Figure 5.
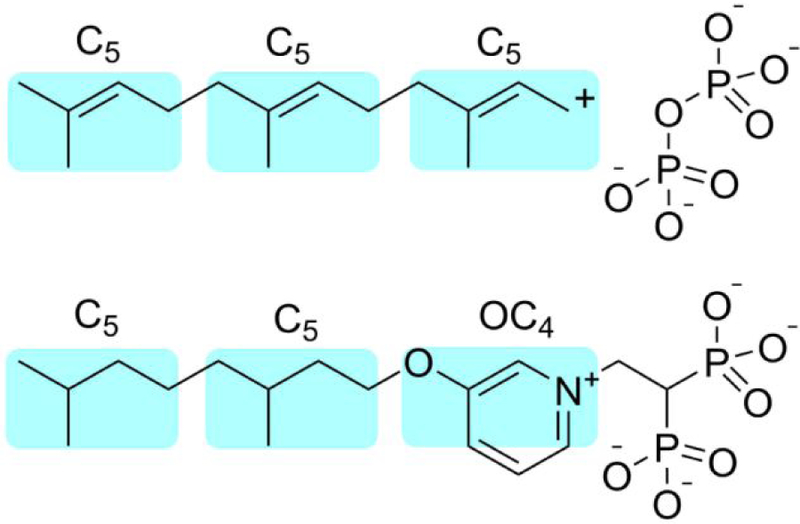
Cartoon illustration of the similarity between a putative FPP transition state/reactive intermediate (top) and a potent SaHepPPS inhibitor, 74 (bottom). Note that this mechanistic proposal would only apply to “long-chain” (~C30, C35, C40) trans prenyltransferases (which use FPP as a substrate) and not to short-chain prenyl transferases, such as FPPS, since FPP is the product and presumably would have only weak binding to FPPS. However, FPP is also known (in human FPPS) to bind to the allosteric (i.e. non-catalytic) FPPS site and acts as an FPPS inhibitor, and it is possible that FPP-analogs may also bind in this way.
