Abstract
Aims
Surgical site infection (SSI) is a common complication of surgery with an incidence of about 1% in the United Kingdom. Sutures can lead to the development of a SSI, as micro-organisms can colonize the suture as it is implanted. Triclosan-coated sutures, being antimicrobical, were developed to reduce the rate of SSI. Our aim was to assess whether triclosan-coated sutures cause a reduction in SSIs following arthroplasty of the hip and knee.
Patients and Methods
This two-arm, parallel, double-blinded study involved 2546 patients undergoing elective total hip (THA) and total knee arthroplasty (TKA) at three hospitals. A total of 1323 were quasi-randomized to a standard suture group, and 1223 being quasi-randomized to the triclosan-coated suture group. The primary endpoint was the rate of SSI at 30 days postoperatively.
Results
The baseline characteristics of age, gender and comorbidities were well matched in the two groups. The rates of superficial SSI were 0.8% in the control group and 0.7% in the intervention group (p = 0.651), and when deep and superficial SSIs were combined the rates were 2.5% and 1.8 (p = 0.266). The length of stay in hospital and the rates of medical complications did not differ significantly between the groups (p = 1.000).
Conclusion
This trial provided no evidence that the use of triclosan-coated sutures at THA and TKA leads to a reduction in the rate of SSI.
Cite this article: Bone Joint J 2018;100-B:296–302.
Keywords: Surgical site infection, Hip arthroplasty, Knee arthroplasty, Triclosan, Triclosan-coated sutures, Antibacterial
More than 180 000 arthroplasties of the hip and knee were performed in 2015 in England and Wales.1 The number of procedures undertaken is rising due to increased availability of healthcare resources, patient demand and the awareness of the benefits related to surgery being undertaken earlier. A study based in the United States has estimated a growth of 174% in total hip (THA) and 673% in total knee arthroplasty (TKA), by 2030.2
Arthroplasties of the hip and knee are generally safe, with low rates of complications.1 One of the most serious complications is surgical site infection (SSI),3 the incidence of which is about 1% in the United Kingdom. This may be an underestimation as higher rates (2.2%) have been reported from centres undertaking active surveillance.4-6 Many factors affect the rate of SSIs and much effort has been concentrated on this challenging area in an endeavour to reduce it.7,8 SSIs are defined as affecting the superficial or deeper tissues handled during THA or TKA,9 according to the Health Protection Agency (HPA). The costs associated with a SSI are escalating annually in the United Kingdom; in 2012, approximate costs were £61 million.10 Frequently, additional societal costs, such as time off work and quality of life are not addressed, which increases the financial burden further. SSI is also seen as a surrogate measure of the quality of health care, with implications for hospitals and providers at a local and national level.11 These factors have reinvigorated enthusiasm at many levels to focus on ways of reducing the rate of SSI.
One such focus is on wound closure, aimed at promoting rapid healing by the apposition of skin edges, preventing the entry of bacteria and leaving a cosmetically acceptable scar.12 During the past century, the material used to close the wound has evolved from natural to synthetic sutures, such as absorbable synthetic fibres. Vicryl (Ethicon-Johnson & Johnson Medical Limited, Kirkton Campus, West Lothian, United Kingdom) is a braided version of this type of suture, which is used ubiquitously across surgical specialities. It elicits little tissue reaction and was one of the first synthetic absorbable sutures to be developed. It is absorbed and retains 65% of its strength 14 days after implantation, 40% of its strength at 21 days and absorption is complete by 70 days.13
An important factor for the development of SSI is colonization of suture material, particularly in knots, which may form a nidus for infection.14 Micro-organisms colonize the suture as it is implanted, potentially developing a biofilm,15 which may subsequently establish immunity to both systemic and local antimicrobial treatment. This has led to an industry aimed at decreasing the risk of colonization of suture material. Previous authors have shown a greater effectiveness of combined systemic and local antibacterial administration.16 Any broad-spectrum local antibacterial agent may be used. However, it must have an established safety profile that does not interfere with the suture material. Triclosan is an established agent that has been effectively used in consumer products for more than 40 years.17 In vitro studies have shown that triclosan-coated sutures create an ‘active zone’ around the suture, inhibiting Staphylococcus aureus, Staphylococcus epidermidis and methicillin-resistant strains of Staphylococcus (MRSA and MRSE), the leading bacteria at the site of surgery, from colonizing on the suture for a minimum of 48 hours.13,18,19
The World Health Organization supports the use of triclosan-coated sutures for the purpose of reducing the risk of SSI.20 The Society for Healthcare Epidemiology of America and Infectious Diseases Society of America practice recommendation is against its routine use, due to unclear evidence.21 The National Institute for Health and Care Excellence report that triclosan may reduce the risk of SSI compared with uncoated sutures22 and will reassess the guidance this year. The interest in triclosan-coated sutures has increased during the last decade with several randomized controlled trials (RCT) assessing its effectiveness. Two meta-analyses have reported contradictory results.23,24
The aim of this study was to determine if using a triclosan-coated suture can significantly reduce the rate of SSI after primary THA or TKA. The 30-day follow-up was the primary outcome measure. We collected data for secondary outcome measures at baseline and at defined timepoints: a) deep incisional infection is defined as a SSI involving the deep tissues (fascial and muscle layers) that occurs within 30 days of surgery if no implant is in place, or within a year if an implant is in place, if the infection appears to be related to the operation, and if the infection meets at least one of the criteria in Table I; b) 30-day and 90-day mortality; c) length of stay (days); d) Clostridium difficile infections; e) complications recorded during the course of the trial; f) critical care admission.
Table I.
Health Protection Agency definition of superficial and deep surgical site infection
| Incisional infection |
|---|
| Superficial incisional infection* |
| SSI that occurs within 30 days of surgery, involves only the skin or subcutaneous tissue of the incision, and meets at least one of the following criteria: |
| 1. Purulent drainage from superficial incision |
| 2. Culture of organisms and pus cells present in fluid/tissue from superficial incision wound swab from superficial incision |
| 3. At least two symptoms of inflammation: a) pain, b) tenderness, c) localized swelling, d) redness, e) heat, and either: f) incision deliberately opened to manage infection, or g) clinician’s diagnosis of superficial SSI |
| Deep incisional infection |
| SSI involving the deep tissues (i.e. fascial and muscle layers), within 30 days of surgery (or one year if an implant is in place), and the infection appears to be related to the surgical procedure and meets at least one of the following criteria: |
| 1. Purulent drainage from deep incision (not organ space) |
| 2. Organisms from culture and pus cells present in fluid/tissue from deep incision or wound swab from deep incision |
| 3. Deep incision dehisces or deliberately opened and patient has at least one symptom of fever or localized pain/tenderness |
| 4. Abscess or other evidence of infection in deep incision: reoperation, histopathology, or radiology |
| 5. Clinician’s diagnosis of deep incisional SSI |
Stitch abscesses (minimal inflammation/discharge at suture point) do not classify as deep surgical site infection (SSI)
Patient and Methods
This study was a three-centre, two-arm, parallel-group, patient-and-assessor-blinded, quasi-randomized controlled trial with block treatment allocation conducted in the United Kingdom. The full details have been described previously,25 and a summary of the methodology follows below. In this pragmatic trial, patients were eligible if they were aged > 18 years, medically fit for an operation and suitable for primary THA or TKA. The surgical approach was determined by the preference of the surgeon.
Patients were recruited between May 2008 and November 2013 from all elective admissions at teaching acute hospital and elective centres. Potential patients were screened and, if eligible, recruited under Good Clinical Practice protocol Inclusion criteria were that the patient was suitable for a THA or TKA, and willing to provide informed consent. Exclusion criteria included revision arthroplasty and patients who were unable to consent. The patients were treated on a standardized enhanced recovery pathway for the whole period of the trial.26 The allocation of treatment was undertaken using opaque envelopes randomized according to the date of surgery. This was based on random monthly assignment into one of the two interventions, each centre providing one form of treatment for a calendar month. It was impossible, for practical reasons, to randomly allocate individuals to treatment groups and the best option was to randomize based on monthly blocks of time.25 Envelopes were opened at the start of a month, so allocation was not known at the time of putting the patient on the waiting list, which was a mean of three months prior to surgery. The participating surgeons were not blinded to the allocation. However, the patients, research team, statistician, clinical staff and associates involved in assessment of outcomes, were all blinded.
The study treatments were standard vicryl suture (control) or triclosan-coated vicryl plus suture (intervention arm). Both sutures used are commercial products, manufactured by the same company (Ethicon, Sommerville, New Jersey). The layer closed with the vicryl was dependent on the preference of the surgeon, ranging from deep fascia to the subcutaneous layer. All vicryl used during the operation was the suture which was allocated. Dressings were standardized in October 2009 to Aquacel Surgical (Convatec, Reading, United Kingdom). Prior to this, it was decided by the preference of the surgeon. All patients entered the same pathway, involving preoperative education, enhanced perioperative management and accelerated discharge.26 Patients had the allocated surgery and all had the same exercises postoperatively in an enhanced recovery program. Unless the operating surgeon specifically advised otherwise, all patients were fully weight-bearing immediately. We did not stipulate the methods of analgesia, anaesthesia or postoperative care. The only deviation from this was a change in prophylactic antibiotics. At the start of trial, the regimen was gentamicin (4.5 mg/kg) and this was changed to gentamicin (3 mg/kg) and teicoplanin (400 mg) on 1 February 2009, in line with our trust guidelines for prophylaxis when undertaking primary arthroplasty.
The primary outcome measure was superficial SSI based on definitions published by the HPA as part of the Surgical Site Infection Surveillance Scheme, which originate from the Centres for Disease Control and Prevention 1992 definition.27 Superficial SSI is defined as an infection that occurs within 30 days of surgery and involves only the skin or subcutaneous tissue of the incision, and meets at least one of the criteria in Table I. We collected data for this outcome up to the 30-day endpoint with telephone follow-up, and patients were monitored for readmission. In order to ensure complete data on SSI that developed after discharge, patients were asked to report problems with the healing of their wound 30 days after the operation using the HPA-designated questionnaire.28 Trained surveillance nurses telephoned patients on or soon after their 30th postoperative day.
A total of 2546 patients awaiting primary THA or TKA were recruited and randomized to the standard care or intervention arms. At the initiation of the study, the combined hospitals’ 12-month audited rate of SSI was 2.5% for THA and TKA. This is in line with other centres in England performing high-quality surveillance using HPA methodology.28 This sample size was calculated based on 80% power to detect a reduction of SSI from 2.5% to 1%, for an uncorrected chi-squared test, at the 5% level, indicating that 1200 patients were need in each group and 2400 in total. This difference represents a significant reduction, which would have an important clinical impact.
Statistical analysis
Baseline demographic and comorbidity data were summarized to check comparability between treatment arms. Additional comorbidities were also recorded that have been shown to increase SSI, such as diabetes29 and rheumatoid arthritis.30 In order to alleviate any possible bias regarding the method of randomization, we undertook formal statistical testing of differences in baseline characteristics between treatment arms to assess whether there was evidence of systematic imbalance introduced by the randomization procedure; independent-samples t-tests and Fisher’s exact test or chi-squared tests were used, with significance set at p < 0.05.
The main analysis assessed differences in the primary endpoint, superficial SSI, on an intention-to-treat basis, between groups using logistic regression analysis of complete case data, adjusting for both the age and gender of the patients. Regression coefficients were significant if p < 0.05. Differences between intervention arms in other secondary outcomes (mortality and critical care stay) and postoperative complications were assessed using chi-squared test or Fisher’s exact test as appropriate. Length of hospital stay was compared between groups using a Mann–Whitney U test. All analyses were undertaken using the statistical software R (R foundation for statistical computing, Vienna, Austria).31
Results
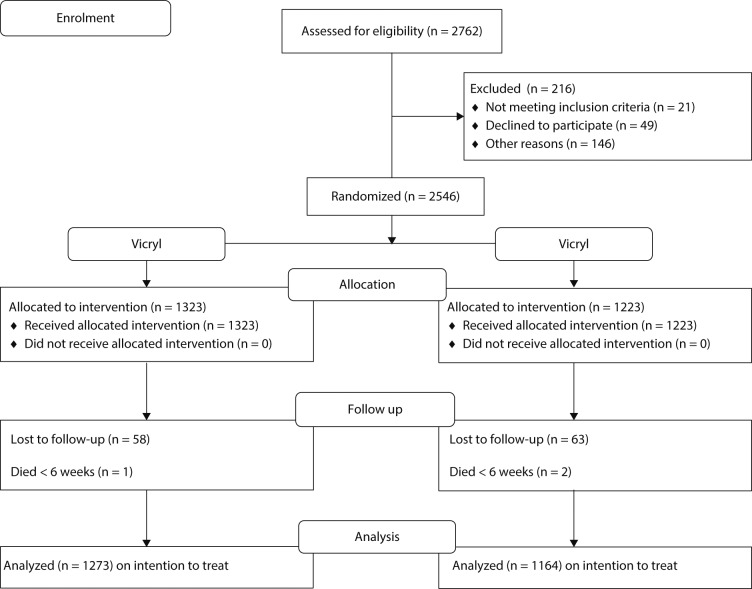
A total of 2762 patients were eligible for inclusion; 216 were excluded. Of these, 146 were not approached or had irregular consent processes, 49 declined to participate and 21 were excluded due to being enrolled in other trials. Thus, 2546 patients consented to take part in the trial. The Consolidated Standards of Reporting Trials diagram shows the flow of these patients through the trial (Fig. 1). All those in the control and intervention arms received the allocated treatment. A total of 1323 were randomized to the standard suture and 1223 to the intervention suture. No patient withdrew consent after being randomized. At the primary endpoint of 30 days, loss to follow-up was < 0.5% in both groups as 2437 patients (95.7%) provided SSI data at this time (Fig. 1). Table II summarizes the demographic characteristics of the patients at baseline. The two groups were well matched and were representative of patients undergoing THA and TKA in the United Kingdom during this period.32 There was no difference in gender distribution (p = 0.879), age (p = 0.564) or any comorbidity between the groups (Table II).
Fig. 1.
Consolidated Standards of Reporting Trials flowchart of patients through the study in the Vicryl (standard suture) and Vicryl plus (triclosan-coated) suture groups.
Table II.
The demographics and comorbidities in the vicryl (standard suture) and the vicryl plus (triclosan-coated) suture groups
| Characteristic | Vicryl (n = 1323) | Vicryl+(n = 1223) | p-value* |
|---|---|---|---|
| Mean age, yrs (sd) | 67.2 (9.7) | 67.5 (10) | 0.564 |
| Gender, female:male (% female) | 719:604 (54.4) | 660:563 (54) | 0.879 |
| Operation, n (%) | 0.782 | ||
| Hip | 590 (46.3) | 532 (45.7) | |
| Knee | 683 (53.7) | 632 (54.3) | |
| Site, n (%) | < 0.001 | ||
| Hexham Hospital | 495 (38.9) | 493 (40.3) | |
| NTG Hospital | 150 (11.8) | 200 (16.4) | |
| Wansbeck General Hospital | 628 (49.3) | 530 (43.3) | |
| Medical history available, n (%) | 1273 | 1164 | |
| Hypertension | 595 (46.74) | 586 (50.34) | 0.082 |
| Atrial fibrillation | 61 (4.79) | 57 (4.9) | 0.979 |
| Ischemic Heart Disease | 93 (7.31) | 81 (6.96) | 0.800 |
| Hypothyroid | 99 (7.78) | 74 (6.36) | 0.199 |
| Type 1 Diabetes | 7 (0.55) | 8 (0.69) | 0.797 |
| Type 2 Diabetes | 135 (10.6) | 109 (9.36) | 0.341 |
| Peripheral Vas Disease | 54 (4.24) | 66 (5.67) | 0.125 |
| COPD | 42 (3.3) | 43 (3.69) | 0.674 |
| Dementia | 1 (0.08) | 1 (0.09) | 1.000 |
| Alzheimers | 2 (0.16) | 3 (0.26) | 0.675 |
| Pressure sores | 2 (0.16) | 0 (0) | 0.501 |
| Psoriatic arthritis | 8 (0.63) | 3 (0.26) | 0.231 |
| Rheumatoid arthritis | 34 (2.67) | 21 (1.8) | 0.193 |
| Hypercholesterlaemia | 70 (5.5) | 75 (6.44) | 0.369 |
Independent sample t-test
NTG, North Tyneside General; COPD, chronic obstructive pulmonary disease
The rate of superficial SSI (Table III) did not differ between the control (0.8%) and intervention (0.7%) groups, based on an intention-to-treat basis. The unadjusted odds ratio was 0.78 (95% confidence interval (CI) 0.27 to 2.15); adjustment for site, operation, age and gender made little difference to the conclusions, with the odds ratio from the logistic regression analysis estimated as 0.78 (95% CI 0.30 to 1.93).
Table III.
The rate of surgical site infection in the two groups, defined according to the Health Protection Agency criteria
| Outcome | Vicryl | Vicryl+ | Total | p-value* |
|---|---|---|---|---|
| Superficial SSI, n (%) | 11/1273 (0.8) | 8/1164 (0.7) | 19/2437 (0.7) | 0.651 |
| Deep SSI, n (%) | 21/1273 (1.6) | 13/1164 (1.1) | 34/2437 (1.3) | 0.300 |
| Deep and superficial SSI, n (%) | 32/1273 (2.5) | 21/1164 (1.8) | 53/2437 (2.1) | 0.266 |
Fisher’s exact test
SSI, surgical site infection
The rate of deep SSI showed no statistical difference, being 1.6% in the control group and 1.1% in the intervention group (p = 0.300) (Table III). When superficial and deep SSI data were combined the rate of infection was 2.5% and 1.8% respectively (p = 0.266) (Table III). Further analyses to compare superficial and deep SSI combined between age groups (2.1% (n = 32) for those aged < 70 years and 2.1% (n = 21) for those aged > 70 years) showed no statistically significant difference. The rate of mortality was not significantly different, being 0.3% in both groups (p = 1.000) (Supplementary table i).
The median length of stay in hospital was not significantly different, being 4.1 days in the control group and 3.9 days in the intervention group (p = 0.386) and there was no difference in the rate of complications between the groups (Table IV).
Table IV.
The postoperative complications in the two groups; p-values are calculated using Fisher’s exact test unless indicated otherwise
| Complication | Vicryl (n = 1273) | Vicryl+ (n = 1164) | p-value† |
|---|---|---|---|
| Deep vein thrombosis, 60 days, n (%) | 4 (0.31) | 7 (0.6) | 0.370 |
| Pulmonary embolism, 60 days, n (%) | 13 (1.02) | 10 (0.86) | 0.834 |
| Stroke, 30 days | 1 (0.08) | 2 (0.17) | 0.609 |
| Transient ischaemic attack, 30 days, n (%) | 0 (0.00) | 0 (0.00) | N/A |
| Gastrointestinal bleed, 30 days, n (%) | 5 (0.39) | 2 (0.17) | 0.456 |
| Renal failure, 30 days, n (%) | 7 (0.55) | 4 (0.34) | 0.552 |
| Urinary retention, 30 days, n (%) | 21 (1.65) | 24 (2.06) | 0.456 |
| Urinary tract infection, 30 days, n (%) | 17 (1.34) | 9 (0.77) | 0.236 |
| Myocardial infarction, 30 days, n (%) | 2 (0.16) | 4 (0.34) | 0.434 |
| Pneumonia, 30 days, n (%) | 9 (0.71) | 6 (0.52) | 0.612 |
| Thrombocytopenia, 30 days, n (%) | 1 (0.08) | 1 (0.09) | 1.000 |
| Ileus, 30 days, n (%) | 0 (0) | 0 (0) | N/A |
| Clostridium difficile, n (%) | 0 (0) | 1 (0.09) | N/A |
| Readmission Clostridium difficile, n (%) | 0 (0) | 2 (0.17) | N/A |
| Blood transfusion, n (%) | 2 (0.16) | 2 (0.17) | 1.000 |
| Readmission, n (%) | 92 (7.23) | 79 (6.79) | 0.730* |
| Aspiration pneumonia, n (%) | 1 (0.08) | 1 (0.09) | 1.000 |
| Low sodium, n (%) | 13 (1.02) | 8 (0.69) | 0.391 |
| Return to theatre (same), n (%) | 3 (0.24) | 2 (0.17) | 1.000 |
| Return to theatre (other), n (%) | 9 (0.71) | 6 (0.52) | 0.612 |
| One or more complications, n (%) | 158 (12.42) | 144 (12.37) | 1.000* |
chi-squared test
Discussion
We found that using a triclosan-coated suture material did not reduce the rate of superficial or overall SSI in primary THA and TKA, in this randomized clinical trial. The groups were equally matched and had statistically similar rates of complications including critical care episodes and comorbidities. All patients received their allocated intervention. No contamination occurred in the crossover phase of the trial, due to the robust method of block randomization.
Some recent meta-analyses have compared the effect of triclosan-coated sutures with standard sutures. In 2012, a comparison of seven RCTs involving 836 patients undergoing a variety of operations concluded that triclosan-coated sutures do not reduce the rates of SSIs or wound breakdown.23 Two further meta-analyses were performed in 2013.33,34 Wang et al33 included 17 RCTs comparing vicryl with vicryl plus in 2160 patients and concluded that triclosan-coated suture had a beneficial effect in the prevention of SSI after surgery. However, this was not specific for orthopaedics. They also recorded that there was not enough information to allow meta-analysis based on detailed individual data.
Recently, Diener et al34 reported the results of a large multicentre, randomized controlled group-sequential superiority trial involving 24 German hospitals and 1224 patients. They concluded that triclosan-coated polydioxanone with triclosan (PDS Plus) did not reduce the rate of SSI after elective midline laparotomy. This represents the largest multicentre RCT in this area and has similar findings to ours, which if combined for a new meta-analysis may change the overall conclusions.
The latest meta-analysis of 21 RCTs by de Jonge et al24 included 6462 patients and was published in 2017. They found that the use of triclosan-coated sutures was associated with a decrease in the rate of SSI. Pooled effects showed a risk ratio of 0.72 (95% CI 0.60 to 0.86; p < 0.001). We recruited 2546 patients into a randomized controlled trial comparing standard vicryl with a triclosan-coated vicryl, which represents a significant proportion of all previous trials. The results of our study and the available literature have not brought about a change in practice at our sites.
This trial has strengths, including its pragmatic design. Although we recruited patients from only three hospitals, the large number of surgeons at various grades involved realistically reflects the wider surgical practice.35 Other strengths included the use of a nationally recognized definition of SSI, which was assessed by HPA-trained nurses, and the high level of follow-up data (95%) at the primary endpoint. The patients, assessors and statistician were blinded to the type of suture which was used. The 100% rate of allocation, which was a further strength, is based on the simplicity of the allocation of treatment, as the sutures for that month were only made available once the randomization had taken place.
The key weakness of the trial was the selected quasi-randomization method, which is widely recognized as being less rigorous than conventional randomization. Differences in the target population, local environment and procedures at each site had the potential to confound the effects of the intervention, so block randomization was used and interventions were randomly assigned using a concealed system on a monthly basis to ensure, as far as possible, that the characteristics of the patients and unknown systematic effects across treatments were balanced. The demographics and important expected comorbidities in the two groups were comparable.
Another limitation was that we did not take into the account the differences in surgical approach between surgeons, nor the grade of the surgeon. The approach could affect closure and the grade of surgeon could affect certain outcomes. In addition to this, the layer at which the vicryl was used was dependent on the preference of the surgeon, and could be any layer from deep layer to subcuticular fascia. However, we believe this variation reflects the pragmatic design of the study and represents the wider surgical practice.
In conclusion, this trial has provided no evidence that triclosan-coated sutures in THA and TKA leads to a reduction in the rate of SSI. Surgeons will be able to use this new information when deciding which type of suture to use when undertaking THA and TKA.
Take home message:
- Triclosan coated sutures do not significantly reduce surgical site infections in elective hip and knee replacement surgery
- This is the first randomised controlled trial assessing the clinical effectiveness of triclosan coated sutures in orthopaedic surgery
Follow M. R. Reed @mikereednhs
Author contributions
A. P. Sprowson†: Conception of the study, Ethics, Government trial clearance, Writing and editing the manuscript.
N. Parsons: Evaluating protocol, Evaluating the statistics, Preparing the data, Preparing the manuscript.
I. Ahmed: Revising the manuscript, Presenting and interpreting the data, Reviewing the literature, Responding to post-submission queries.
C. Jensen: Registering and managing the trial, Leading recruitment, Steering committee, Preparing the manuscript.
P. Partington: Conception of the study, Ethics, Developing the protocol, Preparing the manuscript.
K. Emmerson: Contract negotiation with funder, Institutional clearance, Preparing the manuscript.
I. Carluke: Principle Investigator for site 1, Promoting and updating the trial, Surveillance liaison and reporting, Preparing the manuscript.
S. Asaad: Principle Investigator for site 2, Promoting and updating the trial, Surveillance liaison and reporting, Preparing the manuscript.
R. Pratt: Principle investigator for site 3, Promoting and updating the trial, Surveillance liaison and reporting, Preparing the manuscript.
S. Muller: Designing the trial, Developing protocol, Preparing the manuscript.
M. R. Reed: Chief Investigator, Designing the trial, Steering Committee Chair, Preparing the manuscript.
Funding statement
Registered on the controlled clinical trial database. Registration number: ISRCTN 17807356.
The study has been approved by Newcastle and North Tyneside Research Ethics Committee (07/H0901/62).
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article1
This is an open-access article distributed under the terms of the Creative Commons Attributions license (CC-BY-NC), which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.
This article was primary edited by J. Scott.
Supplementary material
A table showing mortality rates and critical care admission between Vicryl (standard suture) and the Vicryl plus (triclosan coated) suture groups is available alongside the online version of this article at www.bjj.boneandjoint.org.uk
References
- 1.No authors listed. National Joint RegistryRegistry. 13th Annual Report: National joint registry for England, Wales, Northern Ireland and the Isle of Man, 2016. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/13th Annual Report/07950 NJR Annual Report 2016 ONLINE REPORT.pdf (date last accessed 13November2017).
- 2.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg [Am] 2007;89-A:780–785. [DOI] [PubMed] [Google Scholar]
- 3.Makki D, Elgamal T, Evans P, et al. . The orthopaedic manifestation and outcomes of methicillin-sensitive Staphylococcus aureus septicaemia. Bone Joint J 2017;99-B:1545–1551. [DOI] [PubMed] [Google Scholar]
- 4.Heipel D, Ober JF, Edmond MB, Bearman GM. Surgical site infection surveillance for neurosurgical procedures: a comparison of passive surveillance by surgeons to active surveillance by infection control professionals. Am J Infect Control 2007;35:200–202. [DOI] [PubMed] [Google Scholar]
- 5.Wilson J, Wloch C, Saei A, et al. . Inter-hospital comparison of rates of surgical site infection following caesarean section delivery: evaluation of a multicentre surveillance study. J Hosp Infect 2013;84:44–51. [DOI] [PubMed] [Google Scholar]
- 6.Tissingh EK, Sudlow A, Jones A, Nolan JF. Orthopaedic surgical site infection surveillance in NHS England: national audit of current practice. Bone Joint J 2017;99-B:171–174. [DOI] [PubMed] [Google Scholar]
- 7.Rasouli MR, Restrepo C, Maltenfort MG, Purtill JJ, Parvizi J. Risk factors for surgical site infection following total joint arthroplasty. J Bone Joint Surg [Am] 2014;96-A:158. [DOI] [PubMed] [Google Scholar]
- 8.Heller S, Rezapoor M, Parvizi J. Minimising the risk of infection: a peri-operative checklist. Bone Joint J 2016;98-B(Suppl A):18–22. [DOI] [PubMed] [Google Scholar]
- 9.Plowman R, Graves N, Griffin MA, et al. . The rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. J Hosp Infect 2001;47:198–209. [DOI] [PubMed] [Google Scholar]
- 10.Namba RS, Inacio MC, Paxton EW. Risk factors associated with surgical site infection in 30,491 primary total hip replacements. J Bone Joint Surg [Br] 2012;94-B:1330–1338. [DOI] [PubMed] [Google Scholar]
- 11.Gaynes RP, Platt R. Monitoring patient safety in health care: building the case for surrogate measures. Jt Comm J Qual Patient Saf 2006;32:95–101. [DOI] [PubMed] [Google Scholar]
- 12.Reiter D. Methods and materials for wound closure. Otolaryngol Clin North Am 1995;28:1069–1080. [PubMed] [Google Scholar]
- 13.Barbolt TA. Chemistry and safety of triclosan, and its use as an antimicrobial coating on coated vicryl* plus antibacterial suture (coated polyglactin 910 suture with triclosan). Surg Infect (Larchmt) 2002;3(Suppl 1):S45–S53. [DOI] [PubMed] [Google Scholar]
- 14.Sulamanidze M. Evaluation of a novel technique for wound closure using a barbed suture. Plast Reconstr Surg 2007;120:349–350. [DOI] [PubMed] [Google Scholar]
- 15.Kathju S, Nistico L, Tower I, Lasko LA, Stoodley P. Bacterial biofilms on implanted suture material are a cause of surgical site infection. Surg Infect (Larchmt) 2014;15:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rand BC, Penn-Barwell JG, Wenke JC. Combined local and systemic antibiotic delivery improves eradication of wound contamination: An animal experimental model of contaminated fracture. Bone Joint J 2015;97-B:1423–1427. [DOI] [PubMed] [Google Scholar]
- 17.Rodricks JV, Swenberg JA, Borzelleca JF, Maronpot RR, Shipp AM. Triclosan: a critical review of the experimental data and development of margins of safety for consumer products. Crit Rev Toxicol 2010;40:422–484. [DOI] [PubMed] [Google Scholar]
- 18.Rothenburger S, Spangler D, Bhende S, Burkley D. In vitro antimicrobial evaluation of coated vicryl* plus antibacterial suture (coated polyglactin 910 with triclosan) using zone of inhibition assays. Surg Infect (Larchmt) 2002;3(Suppl 1):S79–S87. [DOI] [PubMed] [Google Scholar]
- 19.Storch ML, Rothenburger SJ, Jacinto G. Experimental efficacy study of coated vicryl plus antibacterial suture in guinea pigs challenged with Staphylococcus aureus. Surg Infect (Larchmt) 2004;5:281–288. [DOI] [PubMed] [Google Scholar]
- 20.No authors listed. Global Guidelines for the Prevention of Surgical Site Infection. World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland; 2016. http://www.who.int/gpsc/ssi-prevention-guidelines/en/ (date last accessed 16January2018).
- 21.Anderson DJ, Podgorny K, Berrios-Torres SI, et al. . Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35:605–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.No authors listed. Surgical site infections: prevention and treatment. Updated February 2017. National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/CG74 (date last accessed 13November2017).
- 23.Chang WK, Srinivasa S, Morton R, Hill AG. Triclosan-impregnated sutures to decrease surgical site infections: systematic review and meta-analysis of randomized trials. Ann Surg 2012;255:854–859. [DOI] [PubMed] [Google Scholar]
- 24.de Jonge SW, Atema JJ, Solomkin JS, Boermeester MA. Meta-analysis and trial sequential analysis of triclosan-coated sutures for the prevention of surgical-site infection. Br J Surg 2017;104:118–133. [DOI] [PubMed] [Google Scholar]
- 25.Sprowson AP, Jensen CD, Parsons N, et al. . The effect of triclosan coated sutures on rate of surgical site infection after hip and knee replacement: a protocol for a double-blind randomised controlled trial. BMC Musculoskelet Disord 2014;15:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan SK, Malviya A, Muller SD, et al. . Reduced short-term complications and mortality following Enhanced Recovery primary hip and knee arthroplasty: results from 6,000 consecutive procedures. Acta Orthop 2014;85:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson J, Charlett A, Leong G, McDougall C, Duckworth G. Rates of surgical site infection after hip replacement as a hospital performance indicator: analysis of data from the English mandatory surveillance system. Infect Control Hosp Epidemiol 2008;29:219–226. [DOI] [PubMed] [Google Scholar]
- 28.Leaper D, Tanner J, Kiernan M. Surveillance of surgical site infection: more accurate definitions and intensive recording needed. J Hosp Infect 2013;83:83–86. [DOI] [PubMed] [Google Scholar]
- 29.Turina M, Fry DE, Polk HC Jr. Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med 2005;33:1624–1633. [DOI] [PubMed] [Google Scholar]
- 30.Moucha CS, Clyburn T, Evans RP, Prokuski L. Modifiable risk factors for surgical site infection. J Bone Joint Surg [Am] 2011;93-A:398–404. [PubMed] [Google Scholar]
- 31.Dean CB, Nielsen JD. Generalized linear mixed models: a review and some extensions. Lifetime Data Anal 2007;13:497–512. [DOI] [PubMed] [Google Scholar]
- 32.Jameson SS, Lees D, James P, et al. . Cemented hemiarthroplasty or hip replacement for intracapsular neck of femur fracture? A comparison of 7732 matched patients using national data. Injury 2013;44:1940–1944. [DOI] [PubMed] [Google Scholar]
- 33.Wang ZX, Jiang CP, Cao Y, Ding YT. Systematic review and meta-analysis of triclosan-coated sutures for the prevention of surgical-site infection. Br J Surg 2013;100:465–473. [DOI] [PubMed] [Google Scholar]
- 34.Diener MK, Knebel P, Kieser M, et al. . Effectiveness of triclosan-coated PDS Plus versus uncoated PDS II sutures for prevention of surgical site infection after abdominal wall closure: the randomised controlled PROUD trial. Lancet 2014;384:142–152. [DOI] [PubMed] [Google Scholar]
- 35.Baker PN, Deehan DJ, Lees D, et al. . The effect of surgical factors on early patient-reported outcome measures (PROMS) following total knee replacement. J Bone Joint Surg [Br] 2012;94-B:1058–1066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.