Abstract
Subjective cognitive decline (SCD) represents subjective complaints about cognitive decline in the absence of objective impairment in neuropsychological tests. Recently, growing evidence has suggested that SCD might be the first symptomatic stage of Alzheimer's disease (AD) spectrum disorders. However, SCD is a heterogeneous condition mixed with AD and non-AD related conditions. Hence, refinement of evidence from previous reports and standardization of the concept about SCD are needed to define appropriate target population with AD pathology. In this article, we review previous studies involving subjects with SCD, the new proposed research criteria, and characteristics of SCD in the aspect of preclinical AD. Biomarker status of SCD is also addressed. Future researches on SCD require a longitudinal follow-up with sufficient biomarker studies and proper outcome measures.
Keywords: subjective cognitive decline, Alzheimer's disease, biomarker, preclinical stage
INTRODUCTION
Alzheimer's disease (AD) is the most common cause of dementia and characterized by a slow progression over several years to decades.1,2 Based on the fact that the presymptomatic and prodromal stages of AD exist before the AD dementia stage, new concepts of research criteria for AD have been proposed recently: first, the International Working Group (IWG) proposed 3 stages of AD including the asymptomatic at-risk stage of AD, prodromal AD, and AD dementia.3 Second, The US National Institute on Aging-Alzheimer's Association (NIA-AA) group also proposed 3 stages of AD including the preclinical AD, mild cognitive impairment (MCI) due to AD, and dementia due to AD stages.4 Both criteria include preclinical, asymptomatic stage of AD, in other words, normal cognition with AD-related pathologies, which would be important in terms of a potential target for dementia prevention trials.5 AD-related pathologies in the brain include two pathologic hallmarks, amyloid plaques and tau neurofibrillary tangles.6 They can be assessed using various biomarker tools; for amyloidosis, increased amyloid uptake on amyloid positron emission tomography (PET) and decreased cerebrospinal fluid (CSF) Aβ42 levels can be used; for tau pathologies, tau-related neurodegenerations such as hypometabolism on fludeoxyglucose-PET, brain atrophy on MRI, and increased CSF tau levels are currently used.6 Recently, tau PET scan which can detect in vivo tau depositions is also available. Preclinical AD is a biomarker-based diagnosis which means the presence of positive AD-related pathologic biomarkers with the absence of cognitive impairment on standard neuropsychological tests.6
However, evaluation for AD pathologic biomarkers and selection of preclinical AD from the general populations are hard to achieve. Subjective cognitive decline (SCD) is a state in which the individuals complain of cognitive decline but they have normal performance on standard neuropsychological tests. It is a stage with only subtle neurodegenerative changes but still successful functional compensation, hence, subjects with SCD show cognitive decline which is difficult to detect on standardized cognitive testing and does not reach the level of objective impairment. Recently, SCD might be considered as the first help-seeking and symptomatic stage of AD spectrum disorders based on the biomarker evidence of a temporal lag between the emergence of pathologic Aβ accumulation and the appearance of clinical symptoms.1 After a long and slow rate of presymptomatic change of AD, subtle cognitive decline might be detected only on the subjective level.6 Thus, capturing a valid subjective report about the cognitive decline as an indicator of AD pathology would be rewarding in this population.5 Additionally, biomarker findings indicative of preclinical AD to select high-risk subjects might be critical in studies of SCD.
Previous studies in SCD have many limitations because there were no consensus on the diagnostic criteria and tools to detect the subclinical cognitive changes effectively. Subjective decline in cognition is nonspecific findings and might be related with numerous non-AD related conditions such as normal aging, personality traits, psychiatric disorders, and other neurological or medical conditions.5 Therefore, it is warranted to refine the knowledge about SCD and clarify the risk factors for progression. In this article, we aimed to provide a review of previous study results in subjects with SCD, address the new proposed research criteria, and discuss future directions of studies in this field.
PREVIOUS STUDIES ON SCD
The concept of SCD was first described in 1982.7 Since then, individuals with subjective complaints have been denoted as multiple terms including subjective cognitive impairment, subjective memory impairment, subjective memory decline, and subjective memory failure.
However, there has been no neuropsychological test to detect the subtle cognitive decline in individuals with SCD. Only a few studies investigated whether neuropsychological tests could show the difference between the SCD subjects and normal controls.8,9 Tests for prospective memory function8 or semantic interference9 showed significant differences between normal elderly and SCD subjects.
Previous longitudinal studies reported that individuals with subjective complaints showed an increased risk of future cognitive decline and future progression to MCI or AD dementia.10,11,12,13 Neuroimaging studies revealed cross-sectional evidence of neurodegenerative changes in subjects with SCD in terms of gray matter atrophic changes,14,15,16,17 white matter microstructural changes,18,19,20,21 and functional brain imaging abnormalities.16,22,23 In terms of the gray matter, SCD subjects showed intermediate medial temporal atrophy compared with those of healthy controls and MCI patients, thus mirror the temporal sequence of neurodegeneration in AD continuum disorders.14 A few studies reported that individuals with cognitive complaints showed similar patterns of decreased gray matter with MCI15 or AD17 relative to the healthy controls. Scheef and colleagues reported that subjects with SCD had reduced hippocampal volume compared with normal controls.16 As for white matter microstructural changes, some studies reported similar pattern19 or degree18,20 of microstructural degenerations in subjects with SCD compared with MCI patients. In the perspective of functional brain imaging, a study showed significant hypometabolism in precuneus and it was associated with longitudinal memory decline in the participants.16 Mosconi et al.22 divided SCD subjects into two groups according to the apolipoprotein E (APOE) ε4 existence and showed that ε4 carriers had decreased glucose metabolism in AD-related brain regions and CSF tau/Aβ42 levels similar to AD compared with non-carriers.22 Altered default mode network connectivity in hippocampus of SCD subjects was reported to be in between normal controls and MCI patients.23 These data showed substantial evidence of early AD-related structural and/or functional changes in subjects with SCD and suggested that SCD might be a risk factor for AD dementia. However, they also showed conflicting results with regard to the rate of progression, risk of conversion to MCI or AD dementia, role of baseline biomarkers for detection of AD-related neurodegenerative changes, and pathologic changes. This might be due to the small sample size, lack of common terminology, research criteria, highly variable tools to assess the cognitive complaints, and different research environments.5
PROPOSED RESEARCH CRITERIA OF SCD
SCD-initiative (SCD-I) was launched in 2012 to facilitate the development of a conceptualization for SCD.5 SCD-I suggested a common concept for terminology and conceptual framework to overcome the study limitations in this field.5
‘SCD’ was suggested as a common concept representing normal performance with cognitive complaints. The meanings of the terminology of SCD are as follows: ‘subjective’ refers to the self-perception. ‘Cognitive’ refers to any cognitive domain, not only memory decline because the first symptom of AD may not be limited to memory problem. ‘Decline’ refers to a subjectively experienced worsening of cognition because the terminology reflects the progressive nature of cognitive deterioration in AD continuum disorders.5 In 2014, SCD-I suggested a new research criteria for SCD which must include following conditions, 1) self-experienced persistent decline in cognitive capacity in comparison with a previously normal status and unrelated to an acute event; 2) normal age-, gender-, and education-adjusted performance on standardized cognitive tests which are used to classify MCI or prodromal AD. The exclusion criteria included 1) MCI, prodromal AD, or dementia and 2) can be explained by a psychiatric or neurologic disease (apart from AD), medical disorder, medication, or substance use.5 The diagnostic criteria was regarded as sensitive and potentially overinclusive because specific characteristics of SCD with preclinical AD are not yet established.5
RISK FACTORS OF PROGRESSION IN SCD
SCD-I suggested the characteristic features which increase the likelihood of preclinical AD in subjects with SCD based on the previous evidence in 2014.5 In the article, ‘SCD plus’ representing ‘preclinical AD’ should have following features: subjective decline in memory, rather than other domains of cognition, onset of SCD within the last 5 years, age at onset of SCD ≥60 years old, concerns (worries) associated with SCD, feeling of worse performance than others of the same age group, confirmation of cognitive decline by an informant, presence of the APOE ε4 genotype, and biomarker evidence for AD.5 We previously investigated the most relevant predictors of progression in SCD and combined the predictors with a new modeling scale in order to predict progression based on a nationwide longitudinal cohort data, named Clinical Research Centers for Dementia of South Korea (CREDOS).24 In the study, old age over 60 years, APOE4 carrier, lower Korean version of Mini-Mental State Examination (K-MMSE) recall score below 2, and lower verbal delayed memory score below 50th percentile compared to age-, sex-, and education-specific norms were the most relevant predictors of clinical progression of SCD.24 SCD with more predictors revealed more progression to MCI or AD dementia (HR=5.351, p=0.008).24
Recently, studies using AD-related pathologic biomarkers such as amyloid PET or CSF Aβ1-42/tau levels have been conducted to clarify the most relevant predictors and refine the relationship between subjective complaint and possibilities of preclinical AD.
ASSESSMENT OF SUBJECTIVE COMPLAINTS
Cognitive decline is a common manifestation in healthy older adults and has been reported to be approximately up to 50% of the community-dwelling elderly people.25,26 Considering the diversity of the complaints, various personality traits, and the statistical possibilities of type I error of false positivity, clarifying and quantifying the first-person experience might have some limitations in scientific studies. First, the internal experience of cognitive decline is characteristically complex in their phenomenology and difficult to assess quantitatively.27 Second, self-report measures are largely affected by the subject's factors such as demographics, educational attainment, personality, and mood disorders. Third, too many questions to assess the cognitive complaints might result in type I error rather than true differences.
Assessment of subjective complaint might be divided into two aspects; categorization of the phenomenology and quantification of the complaint. The categorization is needed to define high-risk group with AD-related pathologies. Presenting symptoms, symptoms duration, concerns (worries) associated with cognitive decline, feeling of worse performance than others of the same age group, confirmation of cognitive decline by an informant can be assessed and used for the categorization. The latter, quantification of the complaint, represents the severity of cognitive complaint and can be used to measure the relationship between complaint and biomarker evidence such as neurodegenerative change and AD-related pathologic burden. Rabin and colleagues systemically overviewed the 34 self-report measures currently used in subjects with SCD and refined variability and consistency in the tools.28 Almost all self-report measures were administered in paper-and-pencil format and mainly targeted memory function, followed by executive function.28 Questions about memory function were mostly specific, rather than general, such as items related to memory for the names of people or remembering placement of common objects.28 They demonstrated wide variations in the format, range, time frame, and response options among the measures and the meaning of the complaints might vary according to demographic factors such as educational level and age.28 Thus, in regards of measuring cognitive complaints, careful approaches to the subjects with SCD using a homogeneous measurement tool with a larger sample size that allows a wider range of demographic factors would be needed for future studies.
SCD IN THE ASPECT OF AMYLOID AND TAU BIOMARKERS
Biomarker studies are promising methods for early identification of preclinical AD in SCD subjects, in particular, markers of amyloid and tau accumulation hold promise. However, in contrast to accruing evidence on AD-related biomarkers in subjects with MCI or AD dementia, biomarker use for SCD has not been sufficient yet.
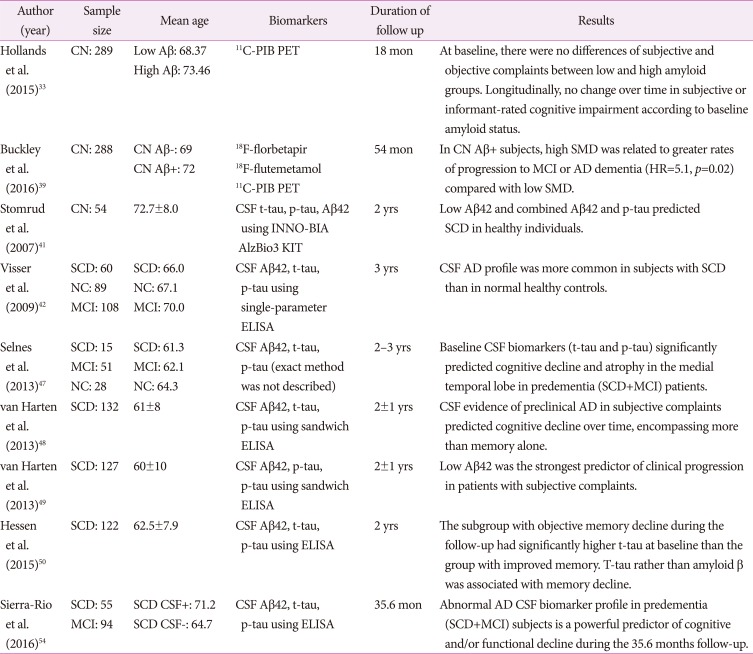
We conducted a literature search using PubMed to overview previously reported biomarker studies using amyloid and tau PET scans or CSF amyloid beta/tau levels in subjects with SCD. We only retrieved English written articles before August 2016. The search terms included ‘biomarker’, ‘amyloid’, ‘amyloid-beta’, ‘tau’ combined with ‘SCD’, ‘subjective memory impairment’, ‘subjective cognitive impairment’, ‘subjective memory failure’, and ‘subjective memory complaint’. Studies that have not shown biomarker results such as amyloid/tau PET or CSF amyloid beta/tau levels were excluded from the review. In total, 28 studies were included in the main review.18,22,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54 We divided the selected studies into three categories; 1) cross-sectional studies using amyloid or tau PET imaging (Table 1), 2) cross-sectional studies using CSF biomarkers (Table 2), and 3) longitudinal studies using PET or CSF biomarkers (Table 3). In summary of the included studies, AD-related biomarker positivity tended to be related with subjective cognitive complaints, however, there was little evidence that AD-related biomarker can differentiate SCD from healthy older adults. Among them, only a few longitudinal follow-up studies investigated the association among subjective complaints, AD-related biomarkers, and cognitive decline or clinical progression to AD dementia.33,39,42,47,48,49,50,54 Moreover, previous longitudinal studies using AD-related biomarkers have limitations in that the follow-up durations were relatively short (ranged from 18 to 54 months) considering that preclinical AD progresses very slowly and two of them included all predementia subjects as well as MCI patients.47,54 Overall, longitudinal biomarker studies suggested that low Aβ42/high tau levels might be the strongest predictor of clinical progression or cognitive decline.47,48,49,50,54 SCD subjects with preclinical AD pathologies either by amyloid PET39 or by CSF47,48,49,50,54 revealed clinical progression or cognitive decline, although one study did not show any significant cognitive decline in follow-up evaluations.42 Which is a more relevant predictor between amyloid or tau pathology has yet to be determined. Some stated that elevated tau levels in CSF would be more important to predict cognitive decline,47,50 because CSF Aβ42 is a very early marker, whereas tau elevation appears to be a marker closer to the actual functional impairment. On the other hand, others concluded that CSF amyloid level is the most relevant predictor in the elderly with normal cognition.48 This should be clarified in the future studies because there has been no prospective long-term follow-up study determining whether baseline amyloid or tau pathology in SCD can predict progression to AD dementia. Our summary of the previous studies regarding biomarker results in SCD is partially consistent with previous reviews.55,56 They qualitatively reviewed previous articles on amyloid and tau biomarkers in SCD and found that AD-related biomarker profiles tend to be more prevalent in subjects with SCD compared with those with healthy controls56 or become increasingly abnormal from SCD to MCI and AD,55 although the results were not always consistent.55,56 The conflicting results might be explained in several ways. First, SCD represents a heterogeneous group. While some may be in the early stage of AD and their cognitive complaints are attributable to underlying AD-related changes, others may not be non-AD conditions. Second, categorizing high versus low amyloid uptake is not always the same.55 Third, there are scanty of tau PET imaging studies, thus the status of tau deposition in SCD could not be clarified yet. Lastly, CSF biomarker results can be affected by analytical and preanalytical factors among the laboratories, resulting in different biomarker values due to interassay and inter-laboratory variation.
Table 1. A summary of cross sectional studies using amyloid/tau PET scans.
APOE: apolipoprotein E, BMI: body mass index, CN: cognitively normal, FDDNP: 2-(1-{6-[(2-[fluorine-18]fluoroethyl)(methyl)amino]-2-naphthyl}-ethylidene)malononitrile, MCI: mild cognitive impairment, NC: normal controls without cognitive complaint, PET: positron emission tomography, PIB: Pittsburgh compound B, SCD: subjective cognitive decline, SMC: subjective memory complaints, SMI: subjective memory impairment.
Table 2. A summary of cross sectional studies using CSF biomarker assay.
AD: Alzheimer's disease, APOE: apolipoprotein E, CSF: cerebrospinal fluid, DR: radial diffusivity, ELISA: enzyme-linked immunosorbent assay, FA: fractional anisotropy, FDG-PET: fludeoxyglucose positron emission tomography, MCI: mild cognitive impairment, NC: normal controls without cognitive complaint, SCD: subjective cognitive decline, SMC: subjective memory complaint.
Table 3. A summary of longitudinal studies using biomarkers.
AD: Alzheimer's disease, CN: cognitively normal, CSF: cerebrospinal fluid, ELISA: enzyme-linked immunosorbent assay, MCI: mild cognitive impairment, NC: normal controls without cognitive complaint, PET: positron emission tomography, PIB: Pittsburgh compound B, SCD: subjective cognitive decline, SMD: subjective memory decline.
FUTURE DIRECTIONS OF STUDIES ON SCD
In spite of the conflicting results of previous studies, individuals of preclinical stage with AD-related pathologies would go through an initial full compensation stage followed by very first decline, thus SCD might serve as the first symptomatic indicator of preclinical AD.5 However, SCD is not identical with preclinical AD. Preclinical AD is a diagnosis based on the biomarker evaluations and does not require the existence of subjective complaints by definition.6 Given that SCD is a heterogeneous condition mixed with preclinical AD and non-AD related conditions, determination of SCD individuals with a high risk of progression, so called preclinical AD, would be important. In light of normal cognitive performance and the worried well of SCD population, subjective complaints in elderly SCD may potentially provide valuable information for early diagnosis of AD spectrum disorders and proper management plan of the disease. To enhance knowledge about specific features of SCD related with preclinical AD, the establishment of universal criteria, assessment tools, and diagnostic guidelines, combined with a biomarker use and longitudinal study design would be warranted. Appropriate selection of SCD with preclinical AD would be useful and practical in preventive clinical trials by enriching the target populations. For the adequate identification, detailed history taking, measuring the severity of cognitive complaints, and characterization of the complaints should be put into practice before going into further biomarker evaluations in the clinical setting.
CONCLUSION
We have reviewed the previous studies, the new proposed research criteria, and the characteristics of SCD in the aspect of preclinical AD. Recent study results using AD-related biomarkers have also been covered. Future researches on SCD require a prospective long-term follow-up with adequate biomarker studies and proper outcome measures to predict and determine the risk of progression in this help-seeking but underestimated group.
Acknowledgements
This study was supported by the grant (2014-0050) from Asan Medical Center.
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 3.Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 4.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reisberg B, Ferris SH, de Leon MJ, Crook T. The global deterioration scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139:1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 8.Rabin LA, Chi SY, Wang C, Fogel J, Kann SJ, Aronov A. Prospective memory on a novel clinical task in older adults with mild cognitive impairment and subjective cognitive decline. Neuropsychol Rehabil. 2014;24:868–893. doi: 10.1080/09602011.2014.915855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loewenstein DA, Curiel RE, Greig MT, Bauer RM, Rosado M, Bowers D, et al. A novel cognitive stress test for the detection of preclinical Alzheimer disease: discriminative properties and relation to amyloid load. Am J Geriatr Psychiatry. 2016;24:804–813. doi: 10.1016/j.jagp.2016.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dufouil C, Fuhrer R, Alpérovitch A. Subjective cognitive complaints and cognitive decline: consequence or predictor? The epidemiology of vascular aging study. J Am Geriatr Soc. 2005;53:616–621. doi: 10.1111/j.1532-5415.2005.53209.x. [DOI] [PubMed] [Google Scholar]
- 11.Glodzik-Sobanska L, Reisberg B, De Santi S, Babb JS, Pirraglia E, Rich KE, et al. Subjective memory complaints: presence, severity and future outcome in normal older subjects. Dement Geriatr Cogn Disord. 2007;24:177–184. doi: 10.1159/000105604. [DOI] [PubMed] [Google Scholar]
- 12.Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kölsch H, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 13.Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27:1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kölsch H, et al. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79:1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- 17.Peter J, Scheef L, Abdulkadir A, Boecker H, Heneka M, Wagner M, et al. Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimers Dement. 2014;10:99–108. doi: 10.1016/j.jalz.2013.05.1764. [DOI] [PubMed] [Google Scholar]
- 18.Stenset V, Bjørnerud A, Fjell AM, Walhovd KB, Hofoss D, Due-Tønnessen P, et al. Cingulum fiber diffusivity and CSF t-tau in patients with subjective and mild cognitive impairment. Neurobiol Aging. 2011;32:581–589. doi: 10.1016/j.neurobiolaging.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Selnes P, Fjell AM, Gjerstad L, Bjørnerud A, Wallin A, Due-Tønnessen P, et al. White matter imaging changes in subjective and mild cognitive impairment. Alzheimers Dement. 2012;8(5 Suppl):S112–S121. doi: 10.1016/j.jalz.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Hong YJ, Yoon B, Shim YS, Ahn KJ, Yang DW, Lee JH. Gray and white matter degenerations in subjective memory impairment: comparisons with normal controls and mild cognitive impairment. J Korean Med Sci. 2015;30:1652–1658. doi: 10.3346/jkms.2015.30.11.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong YJ, Kim CM, Jang EH, Hwang J, Roh JH, Lee JH. White matter changes may precede gray matter loss in elderly with subjective memory impairment. Dement Geriatr Cogn Disord. 2016;42:227–235. doi: 10.1159/000450749. [DOI] [PubMed] [Google Scholar]
- 22.Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, et al. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol Psychiatry. 2008;63:609–618. doi: 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Risacher SL, West JD, McDonald BC, Magee TR, Farlow MR, et al. Altered default mode network connectivity in older adults with cognitive complaints and amnestic mild cognitive impairment. J Alzheimers Dis. 2013;35:751–760. doi: 10.3233/JAD-130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong YJ, Yoon B, Shim YS, Kim SO, Kim HJ, Choi SH, et al. Predictors of clinical progression of subjective memory impairment in elderly subjects: data from the Clinical Research Centers for Dementia of South Korea (CREDOS) Dement Geriatr Cogn Disord. 2015;40:158–165. doi: 10.1159/000430807. [DOI] [PubMed] [Google Scholar]
- 25.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Mewton L, Sachdev P, Anderson T, Sunderland M, Andrews G. Demographic, clinical, and lifestyle correlates of subjective memory complaints in the Australian population. Am J Geriatr Psychiatry. 2014;22:1222–1232. doi: 10.1016/j.jagp.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Abdulrab K, Heun R. Subjective memory impairment. A review of its definitions indicates the need for a comprehensive set of standardised and validated criteria. Eur Psychiatry. 2008;23:321–330. doi: 10.1016/j.eurpsy.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Rabin LA, Smart CM, Crane PK, Amariglio RE, Berman LM, Boada M, et al. Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. J Alzheimers Dis. 2015;48(Suppl 1):S63–S86. doi: 10.3233/JAD-150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodda J, Okello A, Edison P, Dannhauser T, Brooks DJ, Walker Z. (11)C-PIB PET in subjective cognitive impairment. Eur Psychiatry. 2010;25:123–125. doi: 10.1016/j.eurpsy.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50:2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ. Subjective cognition and amyloid deposition imaging: a Pittsburgh compound B positron emission tomography study in normal elderly individuals. Arch Neurol. 2012;69:223–229. doi: 10.1001/archneurol.2011.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amariglio RE, Mormino EC, Pietras AC, Marshall GA, Vannini P, Johnson KA, et al. Subjective cognitive concerns, amyloid-β, and neurodegeneration in clinically normal elderly. Neurology. 2015;85:56–62. doi: 10.1212/WNL.0000000000001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollands S, Lim YY, Buckley R, Pietrzak RH, Snyder PJ, Ames D, et al. Amyloid-β related memory decline is not associated with subjective or informant rated cognitive impairment in healthy adults. J Alzheimers Dis. 2015;43:677–686. doi: 10.3233/JAD-140678. [DOI] [PubMed] [Google Scholar]
- 34.Ivanoiu A, Dricot L, Gilis N, Grandin C, Lhommel R, Quenon L, et al. Classification of non-demented patients attending a memory clinic using the new diagnostic criteria for Alzheimer's disease with diseaserelated biomarkers. J Alzheimers Dis. 2015;43:835–847. doi: 10.3233/JAD-140651. [DOI] [PubMed] [Google Scholar]
- 35.Risacher SL, Kim S, Nho K, Foroud T, Shen L, Petersen RC, et al. APOE effect on Alzheimer's disease biomarkers in older adults with significant memory concern. Alzheimers Dement. 2015;11:1417–1429. doi: 10.1016/j.jalz.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snitz BE, Lopez OL, McDade E, Becker JT, Cohen AD, Price JC, et al. Amyloid-β imaging in older adults presenting to a memory clinic with subjective cognitive decline: a pilot study. J Alzheimers Dis. 2015;48(Suppl 1):S151–S159. doi: 10.3233/JAD-150113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snitz BE, Weissfeld LA, Cohen AD, Lopez OL, Nebes RD, Aizenstein HJ, et al. Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am J Geriatr Psychiatry. 2015;23:985–993. doi: 10.1016/j.jagp.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwan MD, Villemagne VL, Doré V, Buckley R, Bourgeat P, Veljanoski R, et al. Subjective memory complaints in APOEε4 carriers are associated with high amyloid-β burden. J Alzheimers Dis. 2016;49:1115–1122. doi: 10.3233/JAD-150446. [DOI] [PubMed] [Google Scholar]
- 39.Buckley RF, Maruff P, Ames D, Bourgeat P, Martins RN, Masters CL, et al. Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer's disease. Alzheimers Dement. 2016;12:796–804. doi: 10.1016/j.jalz.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Merrill DA, Siddarth P, Raji CA, Emerson ND, Rueda F, Ercoli LM, et al. Modifiable risk factors and brain positron emission tomography measures of amyloid and tau in nondemented adults with memory complaints. Am J Geriatr Psychiatry. 2016;24:729–737. doi: 10.1016/j.jagp.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stomrud E, Hansson O, Blennow K, Minthon L, Londos E. Cerebrospinal fluid biomarkers predict decline in subjective cognitive function over 3 years in healthy elderly. Dement Geriatr Cogn Disord. 2007;24:118–124. doi: 10.1159/000105017. [DOI] [PubMed] [Google Scholar]
- 42.Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund LO, Freund-Levi Y, et al. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619–627. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- 43.Grambaite R, Stenset V, Reinvang I, Walhovd KB, Fjell AM, Fladby T. White matter diffusivity predicts memory in patients with subjective and mild cognitive impairment and normal CSF total tau levels. J Int Neuropsychol Soc. 2010;16:58–69. doi: 10.1017/S1355617709990932. [DOI] [PubMed] [Google Scholar]
- 44.Antonell A, Fortea J, Rami L, Bosch B, Balasa M, Sánchez-Valle R, et al. Different profiles of Alzheimer's disease cerebrospinal fluid biomarkers in controls and subjects with subjective memory complaints. J Neural Transm (Vienna) 2011;118:259–262. doi: 10.1007/s00702-010-0534-0. [DOI] [PubMed] [Google Scholar]
- 45.Rolstad S, Berg AI, Bjerke M, Blennow K, Johansson B, Zetterberg H, et al. Amyloid-β42 is associated with cognitive impairment in healthy elderly and subjective cognitive impairment. J Alzheimers Dis. 2011;26:135–142. doi: 10.3233/JAD-2011-110038. [DOI] [PubMed] [Google Scholar]
- 46.Grambaite R, Hessen E, Auning E, Aarsland D, Selnes P, Fladby T. Correlates of subjective and mild cognitive impairment: depressive symptoms and CSF biomarkers. Dement Geriatr Cogn Dis Extra. 2013;3:291–300. doi: 10.1159/000354188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selnes P, Aarsland D, Bjørnerud A, Gjerstad L, Wallin A, Hessen E, et al. Diffusion tensor imaging surpasses cerebrospinal fluid as predictor of cognitive decline and medial temporal lobe atrophy in subjective cognitive impairment and mild cognitive impairment. J Alzheimers Dis. 2013;33:723–736. doi: 10.3233/JAD-2012-121603. [DOI] [PubMed] [Google Scholar]
- 48.van Harten AC, Smits LL, Teunissen CE, Visser PJ, Koene T, Blankenstein MA, et al. Preclinical AD predicts decline in memory and executive functions in subjective complaints. Neurology. 2013;81:1409–1416. doi: 10.1212/WNL.0b013e3182a8418b. [DOI] [PubMed] [Google Scholar]
- 49.van Harten AC, Visser PJ, Pijnenburg YA, Teunissen CE, Blankenstein MA, Scheltens P, et al. Cerebrospinal fluid Aβ42 is the best predictor of clinical progression in patients with subjective complaints. Alzheimers Dement. 2013;9:481–487. doi: 10.1016/j.jalz.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Hessen E, Nordlund A, Stålhammar J, Eckerström M, Bjerke M, Eckerström C, et al. T-tau is associated with objective memory decline over two years in persons seeking help for subjective cognitive decline: a report from the Gothenburg-Oslo MCI study. J Alzheimers Dis. 2015;47:619–628. doi: 10.3233/JAD-150109. [DOI] [PubMed] [Google Scholar]
- 51.Toledo JB, Bjerke M, Chen K, Rozycki M, Jack CR, Jr, Weiner MW, et al. Memory, executive, and multidomain subtle cognitive impairment: clinical and biomarker findings. Neurology. 2015;85:144–153. doi: 10.1212/WNL.0000000000001738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valech N, Mollica MA, Olives J, Tort A, Fortea J, Lleo A, et al. Informants' perception of subjective cognitive decline helps to discriminate preclinical Alzheimer's disease from normal aging. J Alzheimers Dis. 2015;48(Suppl 1):S87–S98. doi: 10.3233/JAD-150117. [DOI] [PubMed] [Google Scholar]
- 53.Mandecka M, Budziszewska M, Barczak A, PepłońZska B, Chodakowska-Żebrowska M, Filipek-Gliszczyńska A, et al. Association between cerebrospinal fluid biomarkers for Alzheimer's disease, APOE genotypes and auditory verbal learning task in subjective cognitive decline, mild cognitive impairment, and Alzheimer's disease. J Alzheimers Dis. 2016;54:157–168. doi: 10.3233/JAD-160176. [DOI] [PubMed] [Google Scholar]
- 54.Sierra-Rio A, Balasa M, Olives J, Antonell A, Iranzo A, Castellví M, et al. Cerebrospinal fluid biomarkers predict clinical evolution in patients with subjective cognitive decline and mild cognitive impairment. Neurodegener Dis. 2016;16:69–76. doi: 10.1159/000439258. [DOI] [PubMed] [Google Scholar]
- 55.Colijn MA, Grossberg GT. Amyloid and tau biomarkers in subjective cognitive impairment. J Alzheimers Dis. 2015;47:1–8. doi: 10.3233/JAD-150180. [DOI] [PubMed] [Google Scholar]
- 56.Lista S, Molinuevo JL, Cavedo E, Rami L, Amouyel P, Teipel SJ, et al. Evolving evidence for the value of neuroimaging methods and biological markers in subjects categorized with subjective cognitive decline. J Alzheimers Dis. 2015;48(Suppl 1):S171–S191. doi: 10.3233/JAD-150202. [DOI] [PubMed] [Google Scholar]