Chart 1.
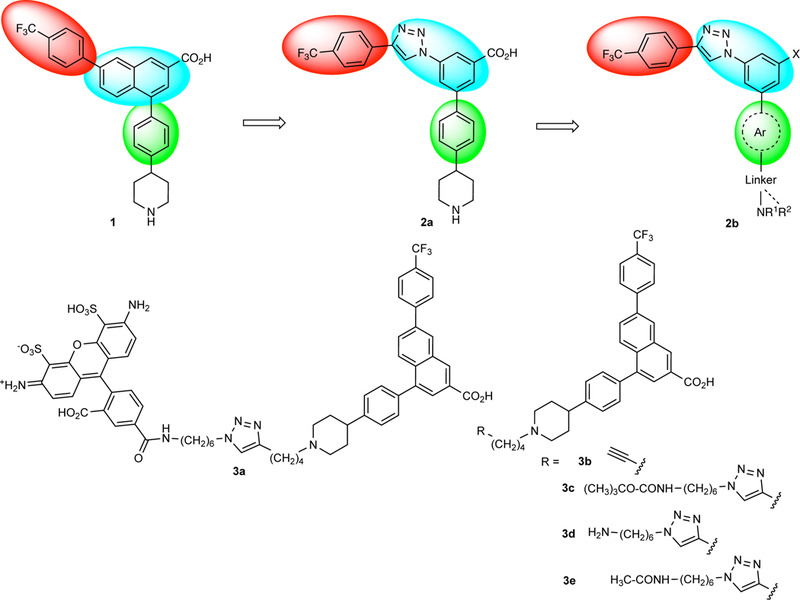
Progression of the structural modifications of naphthoic acid derivative 1 to the present set of derivatives 2b. The major focus was the introduction of diverse heteroaromatic rings in place of the bridging phenyl ring shown in green. The p-trifluoromethylphenyl moiety shown in red and the core benzene ring and adjacent triazole shown in blue were not modified in this study. Compound 3a is the fluorescent antagonist containing structure 1 as a pharmacophore that was used in the flow cytometric assay. Group X is a carboxylic acid or a bioisosteric replacement.
