Figure 2.
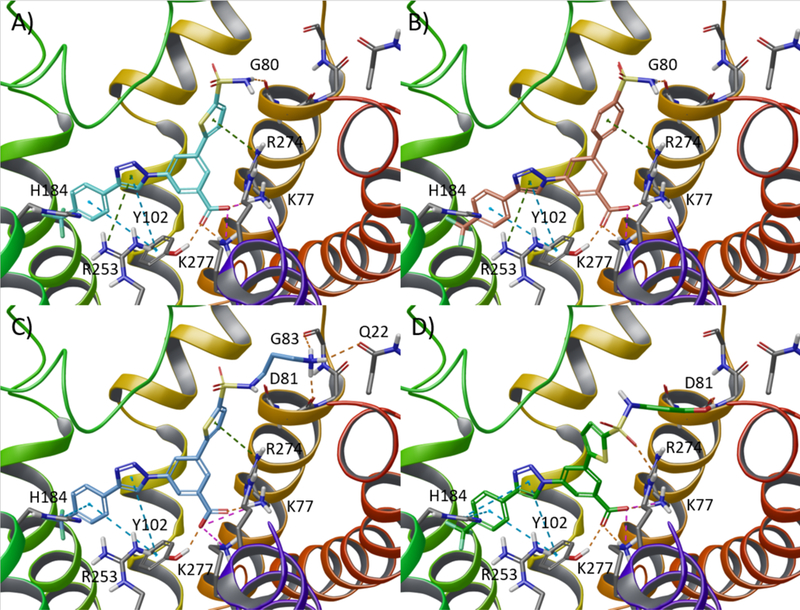
Upper panels: Selection of the aromatic ring. Docking pose of (A) thiophene derivative 13 (cyan carbon atoms) and (B) benzene derivative 16 (salmon carbon atoms) at the hP2Y14R. Both the thiophene and the benzene rings establish an additional π-cation interaction with Arg2747.32 (dark green dashed lines). Lower panel: Selection of the optimal spacer between the aromatic and amine functions. Docking poses of derivatives bearing a spacer of three methylene groups and a (C) terminal primary amine (compound 7, light blue carbon atoms) or (D) a hydroxyl group (dark green carbon atoms) at the hP2Y14R. Side chains of residues important for ligand recognition are reported as sticks (grey carbon atoms). H-bonds, salt bridges, and π-π stacking interactions are pictured as orange, magenta, and cyan dashed lines, respectively. Nonpolar hydrogen atoms are omitted.
