Scheme 5:
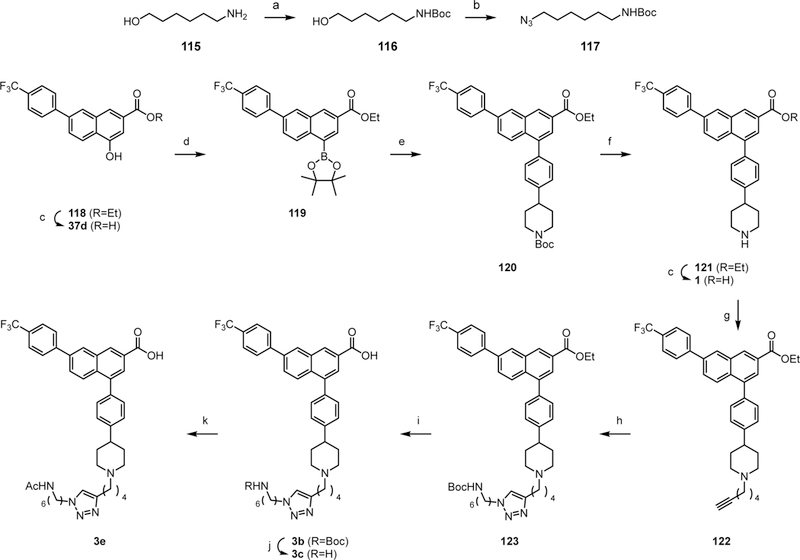
a) Boc2O, Et3N, DCM, rt, 3 h, 80%; b) i) MsCl, Et3N, DCM, 0 °C, 5 min; ii) NaN3, NBu4Br, H2O, 100 °C, 15 h, 59%; c) KOH, MeOH, water, 50 °C, 10 h; d) i) Tf2O, pyr, -78 °C to rt, 1 h; ii) B2pin2, PdCl2(dppf), KOAc, dioxane, 85 °C, 4 h, 61%; e) tert-butyl 4-(4-bromophenyl)piperidine-1- carboxylate 55, Pd(PPh3)4, K2CO3, DMF, 80 °C, 5 h, 59%; f) TFA:THF=1:1, rt, 1 h, 93%; g) 6- bromohexyne-1, K2CO3, DMF, rt, 15 h, 70%; h) 117, CuSO45H2O, sodium ascorbate, DCM:tBuOH:H2O=1:1:1, rt, 15 h, 58%; i) LiOH, MeOH, 80 °C, 15 h, 76%; j) TFA, rt, 30 min, 78%; k) Ac2O, pyr, rt, 1 h, 81%.
