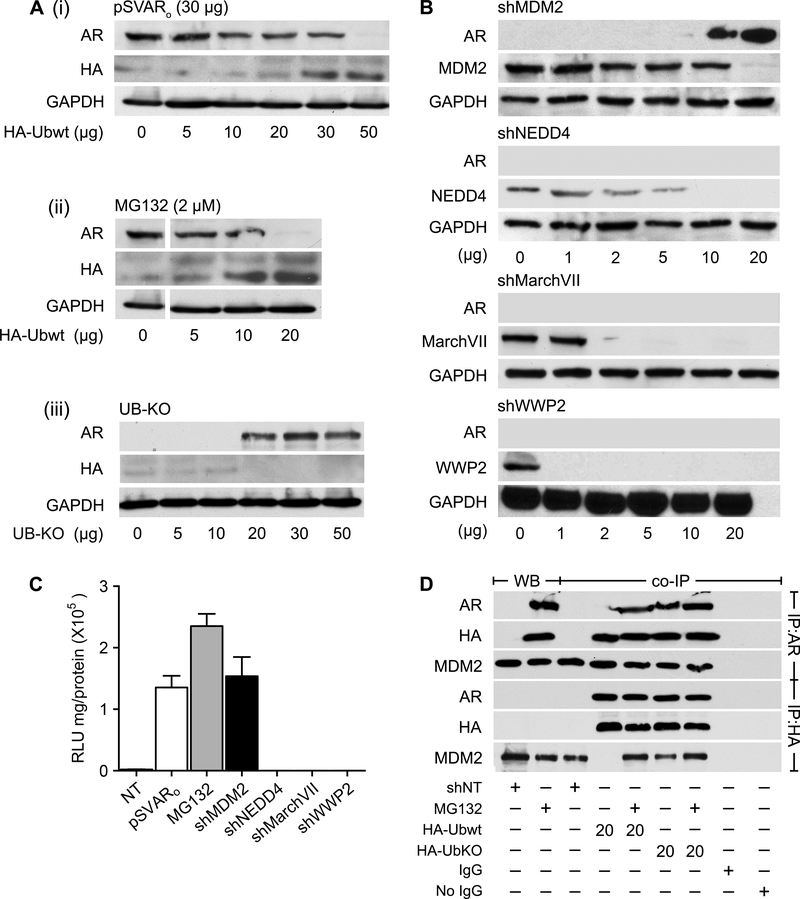
Figure 6. The E3 ligase MDM2 selectively degrades AR in prostate CSCs.
(A) HPET cells were transfected with either 30 μg pSVARo (i) or treated with 2 μM MG132 (ii) and transfected with increasing concentrations of the wild-type ubiquitin expression vector pRK5-HA-Ubiquitin-WT (HA-UbWt) to determine whether poly-ubiquitination regulated AR protein levels. In addition, HPET cells were transfected with increasing concentrations of a mutant ubiquitin plasmid pRK5-HA-Ubiquitin-KO (HA-UbKO, iii) which is incapable of adding ubiquitin molecules onto its target protein, to demonstrate that inhibiting AR poly-ubiquitination would prevent AR degradation.
(B) HPET cells were transfected with increasing concentrations of shMDM2, shNEDD4, shMarchVII or shWWP2 plasmid and protein expression was analyzed by Western blot analysis.
(C) Cells were co-transfected with 20 μg of shMDM2, 10 μg of shNEDD4, 2 μg of shMarchVII or 1ug of shWWP2 plasmid and the ARR2PB-luc and Renilla luciferase reporter gene constructs. Luciferase activity was determined. Positive controls, cells transfected with 30 μg pSVARo or treated with 2 μM MG132. NT, non-targeting shRNA control.
(D) IP analysis was performed to determine whether MDM2 directly binds to AR. Cells were transfected with 20 μg non-targeting shNT control plasmid, 20 μg HA-UbWt, or 20 μg HA-UbKO and treated with 2 μM MG132 as indicated. Antibodies for Western blot analysis are listed in Supplementary Table S1. Data are expressed as mean ± SEM; n=4.