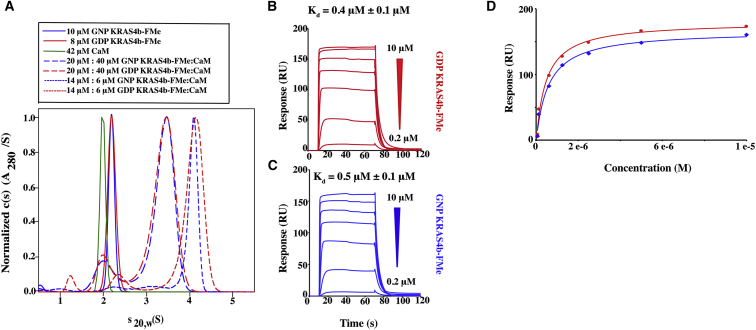
Figure 2.
GDP- and GNP-bound KRAS4b-FMe binds to CaM in a nucleotide-independent manner. (A) Shown are the sedimentation velocity and normalized absorbance c(s) profiles for solutions containing 42 μM CaM (green), 8 μM GDP-bound KRAS4b-FMe (red), and 10 μM GNP-bound KRAS4b-FMe (blue). C(s) distributions for mixtures of 20 μM KRAS4b-FMe and 40 μM CaM as well as 14 μM KRAS4b-FMe and 6 μM CaM are shown as long dash and short dash plots, respectively. Red plots are data for GDP-bound KRAS4b-FMe, and blue plots are for GNP-bound KRAS4b-FMe. (B and C) Shown are SPR binding kinetics of 10–0.2 μM GDP- and GNP-bound KRAS4b-FMe to avi-CaM. Data were fit and yielded Kd values of 0.4 and 0.5 μM, respectively. (D) Fits of the steady-state binding isotherms derived from the SPR data are shown.