Figure 8.
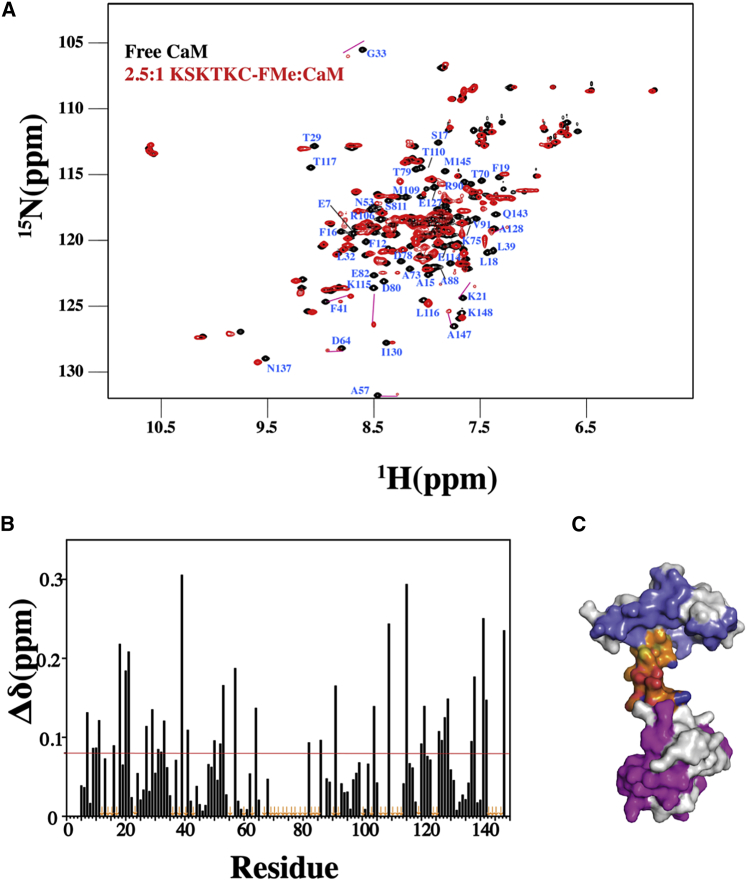
KRAS4b-FMe induces major changes in CaM. (A) 2D 1H-15N TROSY-HSQC spectra obtained of a 15N-labeled CaM (200 μM) in the free (black) and KSKTKC-FMe bound (red states) are shown. The amide signals that exhibited significant chemical shift changes correspond to residues throughout the N- and C-terminal lobes as well as the central linker of CaM. (B) Shown is a histogram of normalized 1H-15N chemical shift changes versus residue number calculated from the HSQC spectra for CaM upon the addition of KSKTKC-FMe. (C) Shown is the surface representation of CaM structure (Protein Data Bank (PDB): 3CLN) highlighting residues that exhibited substantial (>0.1 ppm) chemical shift changes. Residues that were significantly perturbed in CaM-N (blue), central linker (orange), and CaM-C (magenta) regions have been highlighted.