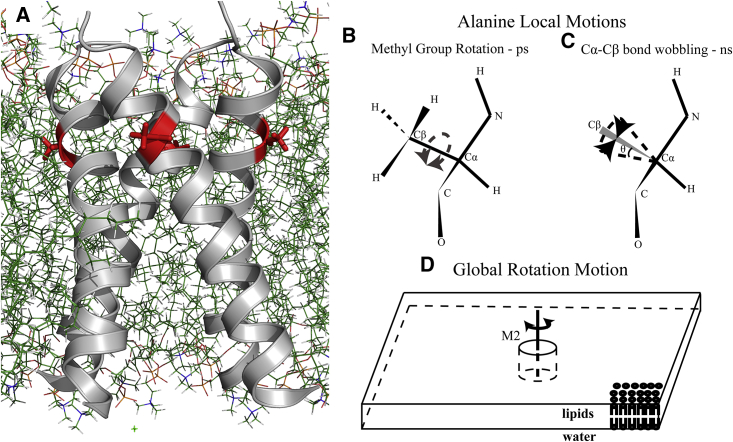
Figure 1.
Different modes of motions of a labeled side chain in the M2TM domain. (A) TM helices of the M2CD structure (PDB: 2L0J) in DOPC/DOPE lipids are shown. In red, Ala29, is shown facing the lipid acyl chains. The 2H NMR spectrum of deuterated Ala29 methyl is affected by three types of motion: (B) methyl rotation about the Cα-Cβ bond on the ps timescale, which can only be damped at temperatures far below 100 K; (C) the wobbling motion of the Cα-Cβ bond, illustrated as the rotation about a cone with amplitude defined by a half angle θ, resulting from small-amplitude motions (libration) of the peptide plane on the ns timescale; and (D) the overall rotational correlation of M2TM, modeled as a cylinder in a two-dimensional slab of lipid bilayer (43).