Figure 1.
Y14 Deficiency Results in Cumulative DNA Damage, Reduced Cell Viability, and Impaired Neurosphere Formation
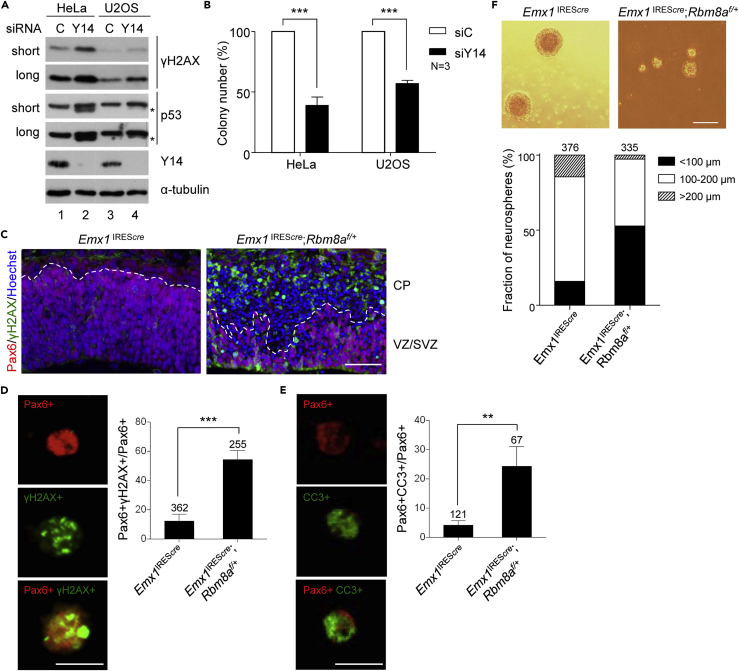
(A) HeLa and U2OS cells were transfected with control siRNA (siC) or siY14. Immunoblotting shows γH2AX and p53 in both short and long exposures and Y14 and α-tubulin. Asterisk indicates p53β.
(B) Clonogenic assay was performed in siRNA-transfected HeLa and U2OS cells. The bar graph shows relative colony-forming units (percentage; mean ± SD). N indicates the number of replicates.
(C) E13.5 dorsal neocortices of Emx1IREScre and Emx1IREScre;Rbm8af/+ mice were subjected to immunostaining using antibodies against γH2AX and Pax6 and Hoechst staining. Dashed line indicates the boundary of the ventricular zone/subventricular zone (VZ/SVZ) and the cortical plate (CP). Scale bar, 50 μm.
(D) Primary cells dissociated from the dorsal neocortices as in (C) were subjected to immunostaining using antibodies against Pax6 and γH2AX as well as Hoechst staining (also see Figure S2F). Representative magnified images show Pax6+, γH2AX+, and double-positive cells of Emx1IREScre;Rbm8af/+ without Hoechst staining. Scale bar in, 10 μm in (D and E). Bar graphs show percentage of γH2AX+ cells among Pax6+ cells (mean ± SD). (D–F) The number of cells analyzed is indicated above the bars; cells were obtained from three pairs of littermates.
(E) As in (D), immunostaining was performed using anti-Pax6 and anti-cleaved caspase 3 (CC3) (also see Figure S1G). Representative magnified images show Pax6+, CC3+, and double-positive cells. Bar graphs show percentage of CC3+ cells among Pax6+ cells (mean ± SD).
(F) Neurosphere formation was performed using dissociated cells from E13.5 dorsal neocortices as in (C) (scale bar, 200 μm). Stacked bar graph shows percentage of different sizes (<100 μm, 100–200 μm, and >200 μm) of neurospheres.
In all bar graphs of Figures 1, 2, 3, 4, 5, 6, and 7, p values are as follows: *p < 0.05, **p < 0.01, ***p <0.001.