Figure 5.
Y14 Depletion Delays the Formation of DNA Damage Foci and Recruitment of DNA Repair Factors
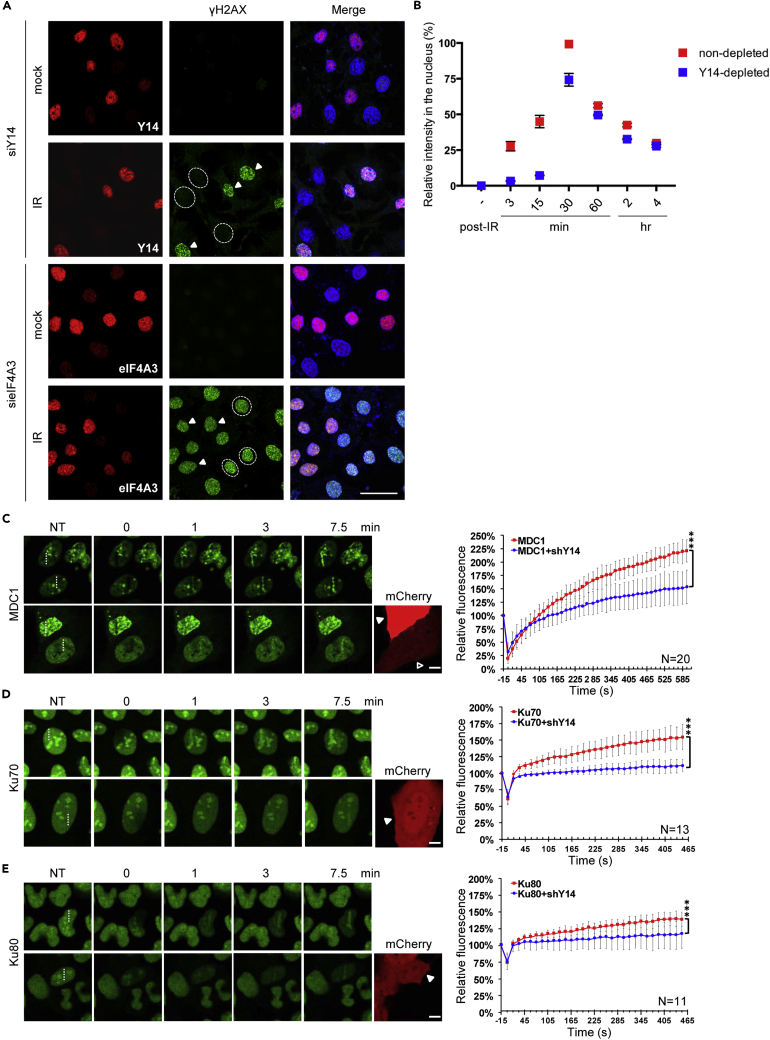
(A) HeLa cells were transfected with an siRNA targeting Y14 or eIF4A3 for 48 h and then left non-irradiated or irradiated at 10 Gy. Cells were fixed 3 min to 4 h (see B) after IR treatment, followed by immunofluorescence using antibodies against Y14, eIF4A3, and γH2AX and Hoechst staining. Representative images show 3-min post-IR. Dashed circle indicate Y14- or eIF4A3-depleted cells, and arrowheads indicate non-transfected cells; three selected cells are indicated. Scale bar, 20 μm.
(B) The experiment was as described in (A). The fluorescence intensities of γH2AX were scored using ImageJ from at least 30 cells per condition. Bar graph shows the relative γH2AX intensity (mean ± SD) of Y14-depleted cells versus non-depleted cells at the indicated time points.
(C–E) U2OS cells that stably expressed the GFP fusion with (C) MDC1, (D) Ku70, or (E) Ku80 were transiently transfected with the Y14 shRNA-mCherry-expressing vectors. Cells were subjected to laser microirradiation (405 nm) followed by live-cell imaging using confocal microscopy. Representative confocal images show accumulation of GFP fusion proteins at sites (dashed lines) of laser microirradiation at the indicated time points. NT indicates samples before microirradiation. The intensity (high, arrowhead; low, empty triangle) of mCherry represents the expression level of shY14. Curve graphs show fluorescence intensities of GFP-fusion proteins at the irradiated region that were quantified periodically, normalized, and presented as mean ± SD for at least 10 cells in each experiment. Scale bar, 10 μm.