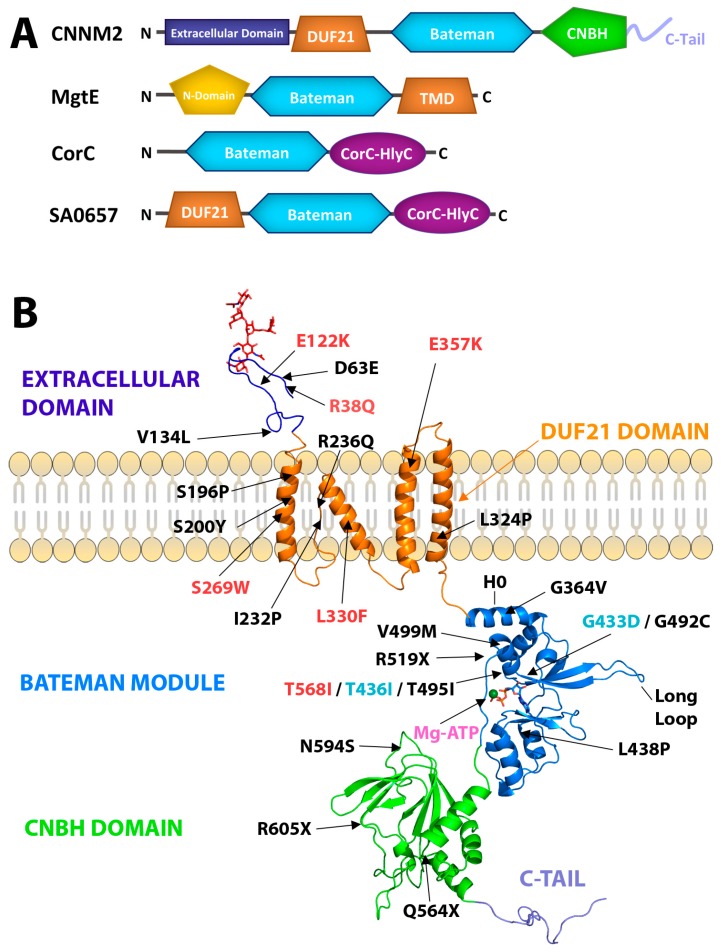
Figure 1.
Structure of CNNMs. (A) Domain distribution in a related cystathionine β-synthase CBS domain containing metal ion transporters, including CNNM2. The Bateman module (in blue) plays a regulatory role in all the proteins shown. The DUF21 domain represents a major part of the transmembrane domain (TMD) (orange) and is thought to contain from three to four α-helices. CNBH domain is in green. The N-terminal extracellular region of CNNM2 is predicted to be β-strand-enriched (deep purple). The bacterial MgtE channel shares the presence of a CorC-HlyC domain (purple) with CorC and with other proteins such as the bacterial SA0657 protein [57]. (B) The panel shows in ribbons the three-dimensional structure of the isolated domains. The structures of the Bateman module and the CNBH domain were extracted from crystallographic data [43,45,58,59]. The representation of the DUF21 domain corresponds to an in silico model [22]. The CNNM representation is as follows: The N-terminal extracellular domain (dark blue) shows the glycosylation in red; the transmembrane α-helixes are represented in orange. In the intracellular region, the Bateman module (PDB code: 4P1O) is represented in blue; bound MgATP is in pink. The CNBH domain (PDB code: 6DJ3) is in green. The unstructured terminal C-tail is in purple. The locations of all known pathological mutations reported for CNNMs are indicated by arrows and in different colors depending on the variant protein: CNNM2 (red), CNNM3 (cyan), and CNNM4 (black). Helix H0 connects the Bateman module with the DUF21 domain.