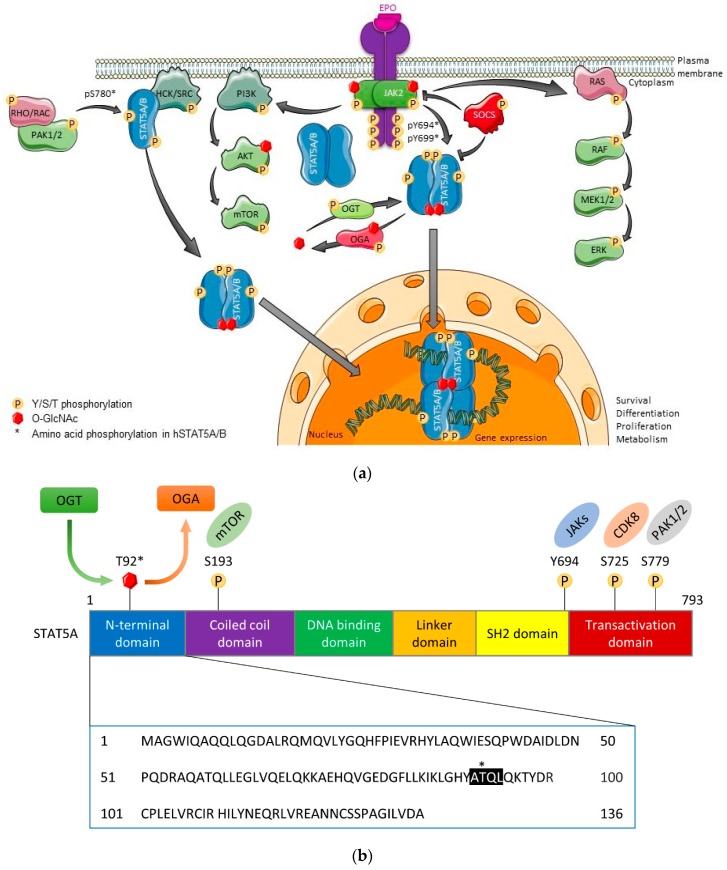
Figure 1.
The JAK2-STAT5-SOCS pathway and domain structure of STAT5. (a) Upon cytokine binding to respective cytokine receptor chain(s), a conformational change in the transmembrane domain triggers activation of associated Janus kinase (JAK) kinases that bind to the BOX1 and BOX2 membrane proximal motifs. Subsequently, antiparallel STAT5 dimers are efficiently recruited and JAK dimers then activate STAT5 by tyrosine phosphorylation. STAT5 then undergoes a drastic conformational change to form parallel dimers. Furthermore, serine/threonine phosphorylation of STAT family members triggers shuttling in and out of the nucleus as well as transcriptional elongation. STATs are usually inactivated by tyrosine phosphatases, which are much more highly expressed than the rate limiting JAK kinases. In contrast, inhibition of cytokine receptor and JAK kinase signaling is executed via ubiquitination by SOCS family members, downstream targets of STATs that provide negative feedback control. JAK2 glycosylation is also observed (our unpublished data). (b) The STAT5A domain structure is schematically illustrated with the O-GlcNAc modification depicted at T92 (indicated by asterisk) within an ATQL motif (highlighted in black) in the N-domain. STATs consist of six domains: N-terminal domain (blue), coiled coil domain (purple), DNA binding domain (green), linker domain (orange), SH2 domain (yellow) and transcriptional activation/stability domain (red) [15,16,19]. Reported phosphorylation sites and respective kinases are also shown. OGT, O-GlcNAc transferase; OGA, O-GlcNAcase.