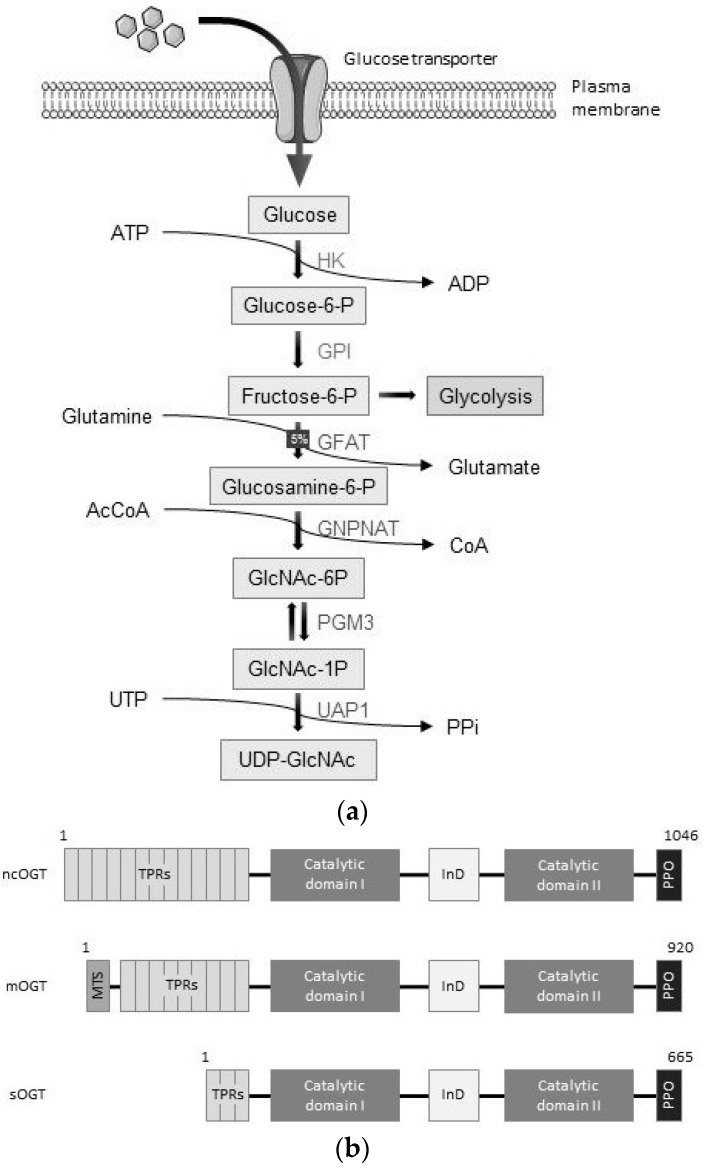
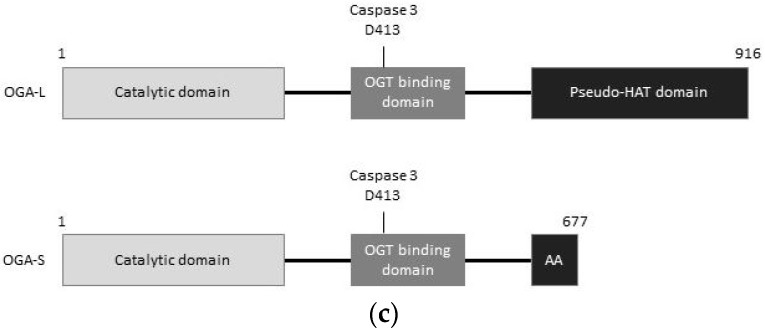
Figure 2.
The hexosamine biosynthetic pathway (HBP) and the two key enzymes: O-GlcNAc transferase (OGT) as a writer and O-GlcNAcase (OGA) as an eraser with their isoform structures. (a) The donor for O-GlcNAcylation, UDP-GlcNAc is synthesized when glucose enters the HBP. Here, glutamine serves as a nitrogen donor and AcCoA serves as an acetyl donor. More detailed pathway descriptions can be found in the text [27,29,35,36]. (b) Three OGT isoforms are known: ncOGT, mOGT, and sOGT. They are generated by alternative splicing and they largely differ in the number of tetratricopeptide repeats (TPRs) at the N-terminus, which is necessary for protein–protein interactions. The catalytic domains I and II are responsible for binding to UDP-GlcNAc and transferring the O-GlcNAc group to serine or threonine residues on target proteins. The OGT enzyme can, for example, be recruited to the cell membrane via the PIP-binding activity domain (PPO) domain as characterized in response to insulin receptor activation by insulin [28,29,37,38]. (c) There are two known isoforms of OGA: a long (OGA-L) and a short (OGA-S). Both share a catalytic domain and an OGT binding domain. The long isoform contains a pseudo-HAT domain, which is located at the C-terminus, and the short isoform has only a short amino acid stretch with less known function. Both isoforms have a cleavage site for caspase 3 at position D413, which is associated with apoptosis [28,29,39].