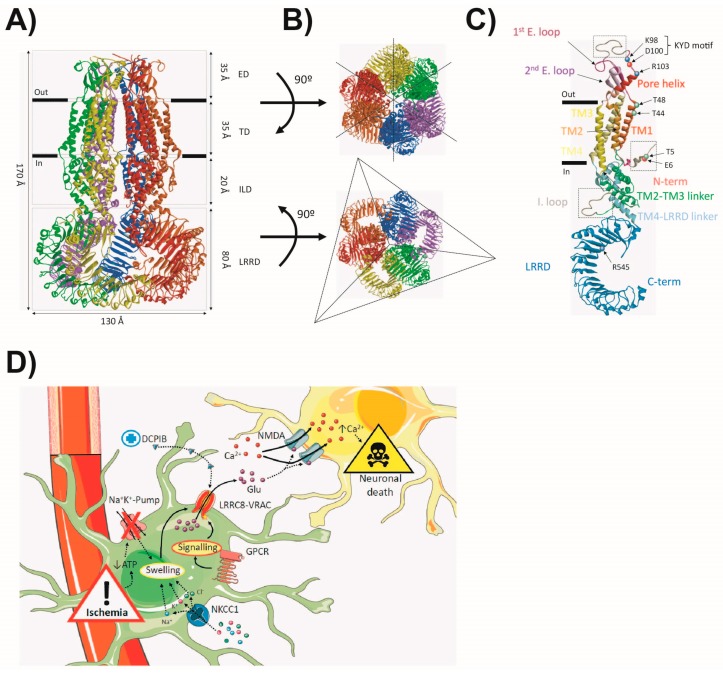
Figure 3.
The VRAC. (A) The cryo-based model of the 8A-VRAC viewed from the membrane plane. The different subunits of the hexamer are highlighted in different colours, and domain layers are indicated by dashed rectangles. (B) Extracellular (top) and intracellular (bottom) views of the 8A-VRAC model. Dashed lines highlight the symmetry axis of the structures, with a six-fold symmetry axis observed in the top view, and a three-fold symmetry axis in the bottom view (LRRD). (C) Representation of a single LRRC8A subunit. Different helices and domains are indicated in different colours. Dashed squares delimit unresolved regions in the structure. Relevant positive residues (blue), negative (red) and polar (green) are indicated with coloured spheres. (D) The hypothetical model of the pathophysiological role of the astrocytic VRAC in ischaemia. Ischaemic conditions lead to depletion of intracellular ATP due to unsustainable oxidative metabolism, triggering the inactivation of the Na+/K+-pump, and subsequently cell swelling. NKCC1 cotransporter activity could further contribute to cell swelling. Astrocyte swelling triggers the opening of the LRRC8-VRAC mediating glutamate efflux. Glutamate build-up in the ischaemic penumbra produces its excitotoxic effect through the overactivation of glutamate-gated channels like NMDA, leading to sustained depolarization and increased Ca2+ levels, thus triggering neuronal damage and death. GPCR signalling could also modulate LRRC8-VRAC activation, but the possible contribution to the process is yet to be discovered. Pharmacological compounds like DCPIB could exert their protective effect by blocking the LRRC8-VRAC, thus reducing glutamate build-up in the ischaemic penumbra.