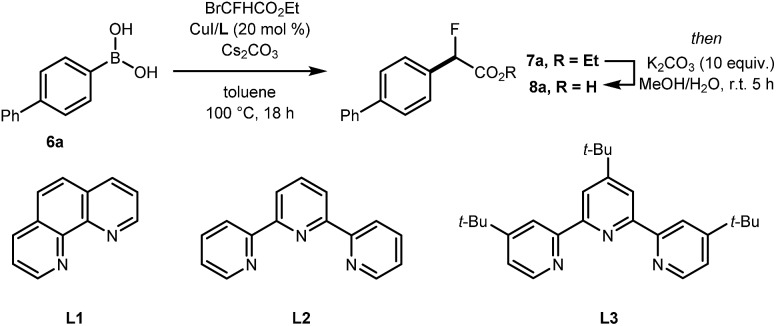
Table 1. Optimisation of the Cu-catalysed cross-coupling of aryl boronic acid 6a with ethyl bromofluoroacetate towards ester 7a and the corresponding carboxylic acid 8a a .
| |||||
| Entry | Solvent | Cu-source | Ligand | Product | Yield b |
| 1 c | Dioxane (0.2 M) | CuI | L1 | 7a | 7% |
| 2 c | Dioxane (0.2 M) | CuI | L2 | 7a | 58% |
| 3 | Toluene (0.2 M) | CuI | L3 | 7a | 63% |
| 4 d | Toluene (0.4 M) | CuI | L3 | 7a | 82% e |
| 5 d | Toluene (0.4 M) | CuI | L3 | 8a | 75% e , f |
| 6 d | Toluene (0.4 M) | CuI | — | 7a | 0% |
| 7 d | Toluene (0.4 M) | — | — | 7a | 0% |
| 8 d | Toluene (0.4 M) | CuCl2 | L2 | 7a | 0% |
| 9 d | DMF or DMSO (0.2 M) | CuI | L3 | 7a | 0% |
aScreening reactions performed on 0.1 mmol scale.
bYield determined by 19F-NMR using α,α,α-trifluorotoluene as internal standard.
c2 equiv. of 6a and 1 equiv. of ethyl bromofluoroacetate.
d1 equiv. of 6a, and 2 equiv. of ethyl bromofluoroacetate.
eYield of isolated product.
fOne-pot procedure towards 8a.
