Table 2. Optimisation studies for the [18F]fluorodecarboxylation of 8b.
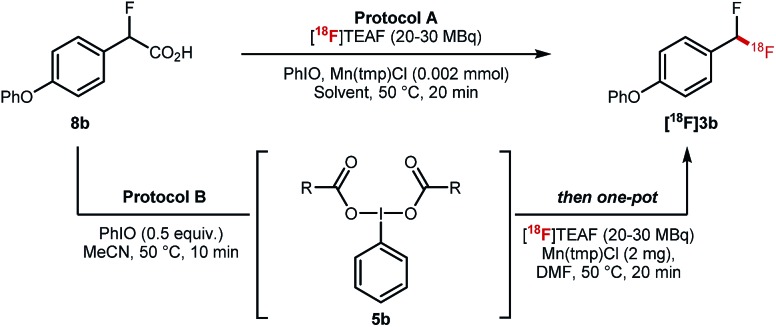
| |||||
| Entry | Starting material (mmol) | Protocol | Solvent | PhIO (mmol) | RCC a , b (n = 2) |
| 1 | 8b (0.11) | A | MeCN c | 0.33 | 3% ± 1% |
| 2 | 8b (0.11) | A | MeCN d | 0.02 | 6% ± 1% |
| 3 | 8b (0.11) | A | DMF d | 0.02 | 7% ± 2% |
| 4 | 8b (0.055) | A | DMF d , e | 0.02 | 22% ± 7% |
| 5 | 5b (0.014) | B | DMF d , e | — | 40% ± 10% f |
| 6 | 5b (0.014) | B | DMF d , e | — | 0% ± 0% g |
| 7 | 8b (0.014) | A | MeCN d | 0.02 | 0% ± 0% h |
| 8 | 5b (0.014) | B | DMF d , e | — | 0% ± 0% i |
aRadiochemical conversion.
b n = number of reactions.
c600 μL of MeCN.
d300 μL of MeCN.
eMeCN removed at 100 °C after dispensing [18F]TEAF.
f(n = 10).
gReaction temperature = 100 °C.
hCatalyst is Mn(tmp)OTs.
iNo Mn Catalyst.
