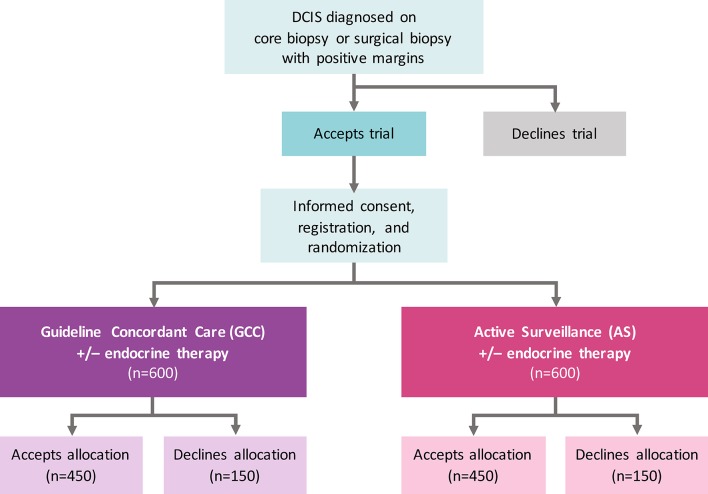
Figure 1.
COMET trial schema. Patient flow for accrual and registration. Eligibility criteria for low-risk DCIS include 40 years of age or older, grade I/II DCIS without invasive breast cancer diagnosed on core, vacuum-assisted or surgical biopsy; ER(+) and/or PR(+); HER2(−); and no mass on physical examination or imaging with exception of fibroadenoma at a distinct/separate site from the site of DCIS. The primary study endpoint on which the sample size is based is rate of 2-year invasive breast cancer diagnosis among patients randomised to GCC compared with AS. ITT analyses adjusted for drop-out, non-compliance and contamination will be performed on all randomised patients including those who do and do not accept the arm to which they are randomised. Patient-reported outcome surveys will be collected from all patients who are registered for the study, including those who crossover. Mammograms will be performed q6 months for the index breast and q12 months for the contralateral breast in the AS arm and q12 months in both the index and contralateral breast in the GCC arm. No chest wall imaging will be performed if mastectomy has been performed. AS, active surveillance; COMET, Comparison of Operative versus Monitoring and Endocrine Therapy; DCIS, ductal carcinoma in situ; ER(+), oestrogen receptor positive; GCC, guideline concordant care; HER2 (−), human epidermal growth factor 2 negative; ITT, intention to treat; PR(+), progesterone receptor positive; q, every.