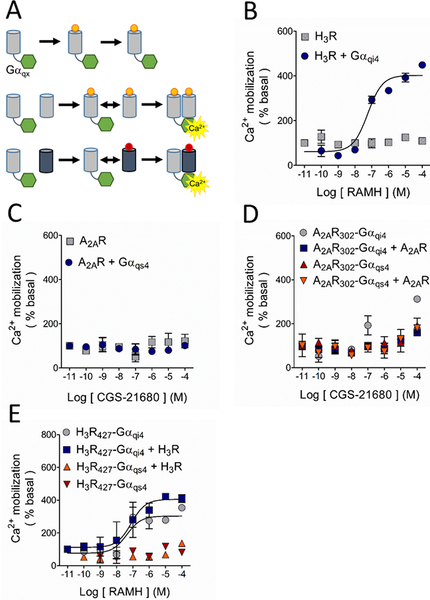
Figure 2.
Study of the possible A2AR-H3R dimerization by functional complementation assay. A. Basis of the assay. The G protein-coupled receptor (GPCR; grey cylinders) is truncated at its carboxyl terminus to generate a nonfunctional GPCR, which is then fused to the chimeric G protein (green hexagons). The chimeric G protein is formed by a Gαq protein in which 9 or 10 residues of the C-terminus were substituted by the corresponding sequence of a Gαs4 or Gαi4 protein (represented by X). Under these conditions, Ca2+ mobilization can only be elicited when the complex GPCR-Gαqx is in close proximity to a wild type (WT) GPCR, either the same (homodimerization) or a different receptor (heterodimerization). B. Activation of the H3R with its agonist RAMH resulted in Ca2+ mobilization only when it was co-expressed with the chimeric Gαqi4 but not when transfected alone. C. When activated with its agonist CGS-21680, the A2AR was uncapable to induce Ca2+ mobilization either alone or when co-transfected with the chimeric Gαqs4 protein. D. Functional complementation by homodimerization of the truncated A2AR302, either bound to Gαqs4 (A2AR302-Gαqs4) or Gαqi4 (A2AR302-Gαqi4), with the native A2AR was not observed. E. Activation of the truncated H3R427 bound to Gαqi4 (H3R427-Gαqi4) induced Ca2+ mobilization on its own and homodimerization with the WT-H3R did not modify the response. No signal was observed with activation of the H3R427-Gαqs4 construct when it was co-expressed with the WT-H3R. In all graphs data are means ± SEM from 3 replicates from a representative experiment. Where SEM bars are not visible, they are smaller than the symbol size. The quantitative analysis is shown in Tables 1 and Supplementary Table 2.