Abstract
Rapid economic growth and urbanization is imposing an unseen pressure on energy sector to fulfill the increasing energy demand. Non edible horticultural residues viz. wheat and pearl millet straw have the potential to become an economical resource for waste to energy conversion. However, maximum hydrolyzability of the crop residues is a prerequisite for efficient conversion of complex organic materials into biofuels. In the present study, mycological treatment of wheat and pearl millet straw was accomplished by employing Chaetomium globosporum. The straw samples were exposed to mycological treatment for 14, 28 and 42 days. The improvement in hydrolyzability of straw was assessed by estimating the increase in reducing sugar release. The competence of Chaetomium globosporum for treating the straw samples was evaluated by measuring the % lignin removal after treatment. Furthermore, the structural and morphological changes in the straw samples after mycological treatment were examined by using scanning electron microscopy, Fourier transform infrared spectroscopy and X-ray diffraction analysis. The results revealed 124 and 91% increase in reducing sugar release along with 43 and 41% removal of lignin for wheat and pearl millet straw respectively. Significant differences were also observed in in the structure, crystallanity and surface morphology.
Keywords: Microbiology, Biotechnology, Environmental science
1. Introduction
Prompt exhaustion of conventional energy resources, unbalanced demand supply patterns and raised concern over greenhouse gas emissions have directed the researchers to delve into the alternative renewable resources for harnessing energy [1]. The lignocellulosic agriculture residues viz wheat straw, pearl millet straw, corn straw etc. can be a suitable feedstock for production of bio-methane through the process of anaerobic digestion (AD). However, the conversion of waste residues to biogas relies upon the bioavailability of cellulose for enzymatic hydrolysis [2]. The hindrance in enzymatic hydrolysis is usually imposed by the polyphenolic lignin component of crop residues which is non-degradable due to the presence of covalent and non-covalent cross linkages [3]. Therefore, various pretreatment strategies are employed for degrading the recalcitrant layer of lignin and making the lignocellulosic waste residues readily hydrolysable. The pretreatment of biomass can be accomplished by employing chemical (acid, alkali, ionic liquids etc.), mechanical (milling, hot water, irradiation, ultrasonic etc.) and biological approaches (fungal and bacterial) [1, 4, 5]. Out of these pretreatment strategies, the biological pretreatment is the most energy efficient, environmental friendly and economically viable approach which imitates the degradation process of lignin existing in nature [6, 7]. Several bacterial and fungal species have been reported to possess the lignin degrading enzyme system which degrades the phenolic and non-phenolic components of lignin [5, 8].
In the present study, the mycological treatment of lignocellulosic biomass i.e. wheat and pearl millet straw was carried out by employing Chaetomium globosporum. Chaetomium globosporum has not been explored earlier for its capability to act as lignolytic fungus. So far, application of Chaetomium globosporum has only reported for production of bioactive metabolites [9]. There is only one study regarding the application of Chaetomium globosporum for biological pretreatment [10]. The impact of treatment on the degradability of biomass was analyzed in terms of the quantity of released reducing sugar after treatment and the change in chemical composition of lignin, cellulose and hemicellulose.
2. Material and methods
2.1. Raw material
Wheat and pearl millet straw were gathered from the Bhurthal village in Jaipur district of Rajasthan in India (26° 59′ 47″ N and 75° 52′ 35″ E). Collected straw samples were subjected to milling and sieving to obtain uniform size (5 mm). The milled straw samples were stored in air tight packets at room temperature till the time of experiment. For isolation of Chaetomium globosporum, the soil samples were collected from hot semi-arid zones of Bikaner, Rajasthan, India.
2.2. Isolation of Chaetomium globosporum
Isolation of Chaetomium globosporum was carried out by employing growth media in which lignin was provided as the exclusive source of carbon. The chemical composition of the growth media broth consisted of NaNO2: 2 g; K2HPO4: 1 g; NaCl: 0.5 g; FeSO4: 0.01; lignin: 0.1 g per liter and pH 6.0 ± 0.3 [11]. The broth was inoculated with the soil samples and incubated for 7 days (120 rpm, 28 °C). Thereafter, the grown fungal suspension was used to inoculate potato dextrose agar (PDA) Petri plates employing spread plate method. The Petri plates were subcultured periodically till the pure colonies of Chaetomium globosporum were obtained. Identification of fungus was carried out at Indian Type Culture Collection (ITCC), Indian Agricultural Research Institute, New Delhi, India. The pure isolated strain of Chaetomium globosporum was maintained on PDA Petri plates and slants and stored at 4 °C.
2.3. Screening of lignolytic ability of fungi
Quantitative analysis of lignolytic activity was performed by determining the laccase activity in the fermentation media. Media composition consisted of NaNO2: 0.02 g, MgSO4.7H2O: 0.005 g, K2HPO4 0.01 g, NaCl: 0.005 g, FeSO4.7H2O: 0.001 g, ZnSO4: 0.0001 g, CuSO4: 0.0001 g, Sucrose: 0.3 g per litre at pH 6.0 [11]. Guaiacol (0.02%, 0.01 mL) was supplemented to the media for inducing the secretion of laccase by the fungus. Laccase activity of the culture broth was monitored for 7 days. Aliquots were withdrawn at regular interval of 24 h till 7 days and laccase activity was measured as per procedure employed by Monssef et al. [12]. The reaction mixture for laccase activity estimation by guaiacol assay consisted of 1 mL of 2 mM guaiacol solution, 3 mL of 10 mM sodium acetate buffer solution and 1 mL of fungal culture supernatant. Absorbance of the reaction mixture was measured at 450 nm using UV spectrophotometer (Shimadzu-1800, Japan). Activity of laccase was expressed in international units (IU), where 1 IU referred to the quantity of enzyme essential for oxidizing 1 μmol of guaiacol per min.
2.4. Mycological treatment of biomass
For performing the mycological treatment, 5 g of straw samples were taken into 250 mL Erlenmeyer flask. Distilled water was supplemented to the straw samples for obtaining the moisture content of 85%. Thereafter, the flasks containing the straw samples were cotton plugged and sterilized by autoclaving for 20 min at 15 lbs pressure and 121 °C. The autoclaved flasks containing straw samples were cooled down to room temperature, followed by inoculation with 5 discs of fungal mycelia (4 mm diameter) procured from full grown PDA plates of Chaetomium globosporum. Flasks which were not inoculated with fungal mycelia were taken as control samples. Control samples have the same quantity of water added to inoculated flasks. Thereafter the flasks (both control and inoculated) were incubated at 28 °C for time periods of 14, 28 and 42 days. On completion of the stipulated treatment period, aliquots were withdrawn from the flasks for estimating the quantity of released reducing sugars and laccase activity.
The released reducing sugar were measured by using dinitrosalycilic acid (DNS) method. The reaction mixture comprised of 2 mL sample and 2 mL of DNS followed by heating at 90 °C for 20 min. Thereafter, the reaction mixtures were cooled down to room temperature and 40% sodium potassium tartrate was added to stabilize the color of the solutions. Absorbance of reaction mixtures was measured at 540 nm and reducing sugar concentration was calculated by comparing with the standard calibration curve of glucose [13]. The reducing sugars in control samples were also quantified and compared with the pretreated samples to neglect the effect of water solubilization on straw samples. The % increase in reducing sugars in pretreated samples as compared to control samples was determined. Laccase activity was measured by employing the guaiacol assay method described as earlier in section 2.3.
2.5. Characterization of biomass
Surface morphology of untreated and treated straw samples was analyzed by using scanning electron microscopy (SEM) to observe the morphological changes occurred in the straw samples after treatment. The straw samples were mounted on aluminum stubs. Gold coating was performed to make the samples conductive. Nova NanoSEM 450 electron microscope (Nova, Netherlands) was employed to record the SEM images. Scans were performed at 20 kV and 1000 X magnification. Fourier transform infrared spectroscopy (FTIR) was carried out by using Spectrum 10.4.00 FTIR spectrophotometer (PerkinElmer, USA) to reveal the changes occurred in the functional groups present in the straw samples. FTIR was performed by preparing potassium bromide (KBr) pellets of sample. For pellet preparation, straw samples were converted to fine particles and mixed with KBr. The mixture was put in pellet die set and subjected to hydraulic press for pellet formation. FTIR spectra of sample pellets were recorded from 400 to 4000 cm−1 wave number. Percentage crystallinity (% Cr) and crystallinity index (C.I.) of untreated and treated straw were studied and compared using an X-ray diffractometer (XPERT-PRO, UK). The samples were converted in powder form and mounted on glass slides. XRD was performed using Cu Kα radiation source with varying (5-50°) diffraction angle (2θ). The of lignocellulosic biomass is strongly associated with its chemical composition. During determination of C.I. the cellulose was considered as crystalline while lignin and hemicellulose components were considered as amorphous [6]. Any change in C.I. represented changes in the composition of cellulose, hemicellulose and lignin. The % Cr in samples and its C.I. was calculated from the following equations:
| Eq. (1) |
| Eq. (2) |
Where I22 is the intensity of crystalline peak at 2θ = 22° and I18 is intensity of amorphous peak at 2θ = 18° [6].
2.6. Chemical composition analysis
The assessment of changes occurred in chemical components of straw i.e. lignin, cellulose and hemicellulose after mycological treatment was based on the method elaborated by Harper and Lynch [14]. The quantification of these three major components of straw samples was accomplished by the sequential extraction and weighing process. Firstly, hot water soluble materials were quantified by boiling the straw samples in water for 1 h followed by overnight drying at 60 °C. The weight loss in straw after drying was considered as hot water soluble materials. The remaining straw was subjected to lignin estimation by submerging in 30 mL water solution having acetic acid (10%) and 0.6 g sodium chlorite. The solution comprising straw was heated for 1 h at 75 °C. Thereafter acetic acid and sodium chlorite were added again to the solutions and heating extended for 2 h. The samples were then neutralized by washing with water, acetone and ether before drying (105 °C, 90 min). The weight loss in the samples after drying was reflected as the weight of lignin. For hemicellulose estimation, the remaining straw after lignin estimation was subjected to 20 mL of 24% KOH. The samples were then neutralized by washing with acetic acid (5%), water, acetone and ether before drying (105 °C, 90 min). The weight loss was taken as the quantity of hemicellulose while the remaining weight was considered as cellulose.
3. Results and discussion
3.1. Isolation of Chaetomium globosporum and screening of lignolytic ability
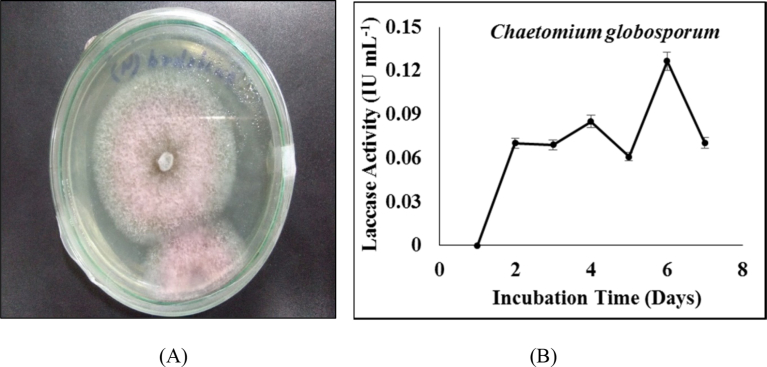
Chaetomium globosporum was isolated from the soil samples collected from the multiple sites of Bikaner (Fig. 1(A)). Laccase activity of the strain in fermentation media was monitored till 7 days. As shown in Fig. 1(B), the highest activity of laccase was observed on 6th day (0.127 IU mL−1). Drop in laccase activity was observed at 5th day which may be due to the interference of other carbohydrate active enzymes secreted by fungus. It is reported in earlier studies that the activity of enzymes at particular day is affected by the presence and activity of other enzymes secreted by the organism [6].
Fig. 1.
(A) Isolated pure colony of Chaetomium globosporum (B) Laccase activity of Chaetomium globosporum in fermentation media.
3.2. Impact of mycological treatment
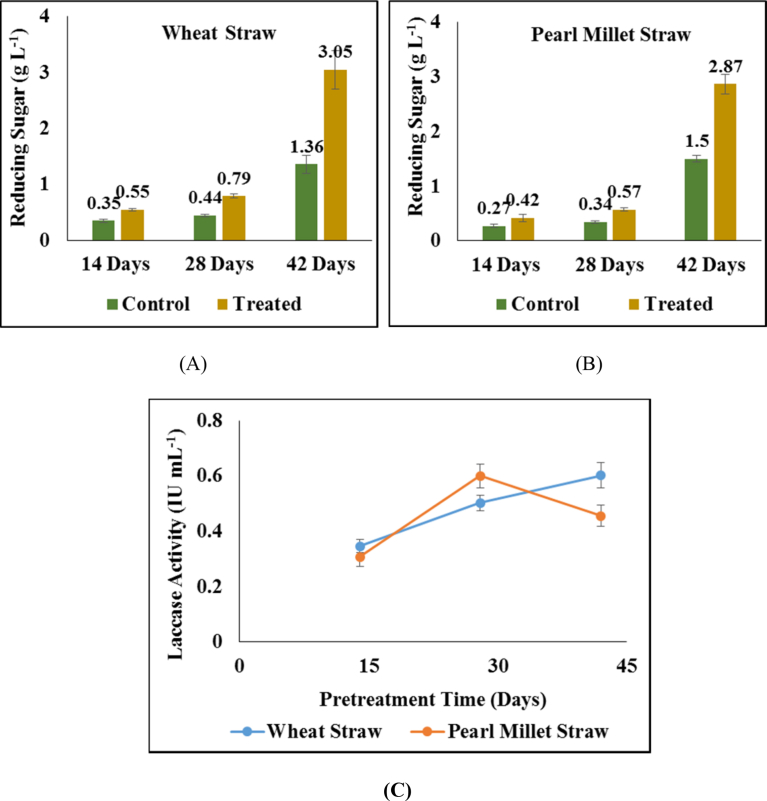
The impact of mycological treatment on the quantity of released reducing sugars is depicted in Figs. 2(A) and 2(B). As shown in figures the amount of reducing sugars increased as the duration of treatment increases. Even though the increase in reducing sugar was observed in the control samples also. However, the increment observed in treated samples was much higher as compared to the control samples. The increased sugar yield in the control samples depicted the effect of water on the straw where water solubilized the unbound sugar present in the untreated straw samples. Still, the much higher increase in the treated samples during the same time duration attributed to the breakdown of lignin barrier in the treated straw samples leading to higher availability of free sugars as compared to their untreated counterparts. After 28 days of treatment the improvement in reducing sugar yield was estimated to be 79% and 67% for wheat and pearl millet straw respectively. While after 42 days of treatment, the reducing sugar yield enhanced by 124% and 91% in wheat and pearl millet straw respectively. The enhancement in the amount of reducing sugars with treatment time was in agreement with the laccase activity measured in treated wheat straw after 14, 28 and 42 days (Fig. 2(C)). However, in the pearl millet straw, the laccase activity decreased after 28 days. Still, the reducing sugar yield continued to enhance. This may be attributed to the activity of cellulase enzyme secreted by the fungus which have higher access to cellulose after the breakdown of lignin barrier by laccase enzyme during 28 days of treatment.
Fig. 2.
Impact of treatment on (A) reducing sugar release in wheat straw; (B) reducing sugar release in pearl millet straw and (C) laccase activity during treatment.
3.3. Structural characterization of biomass
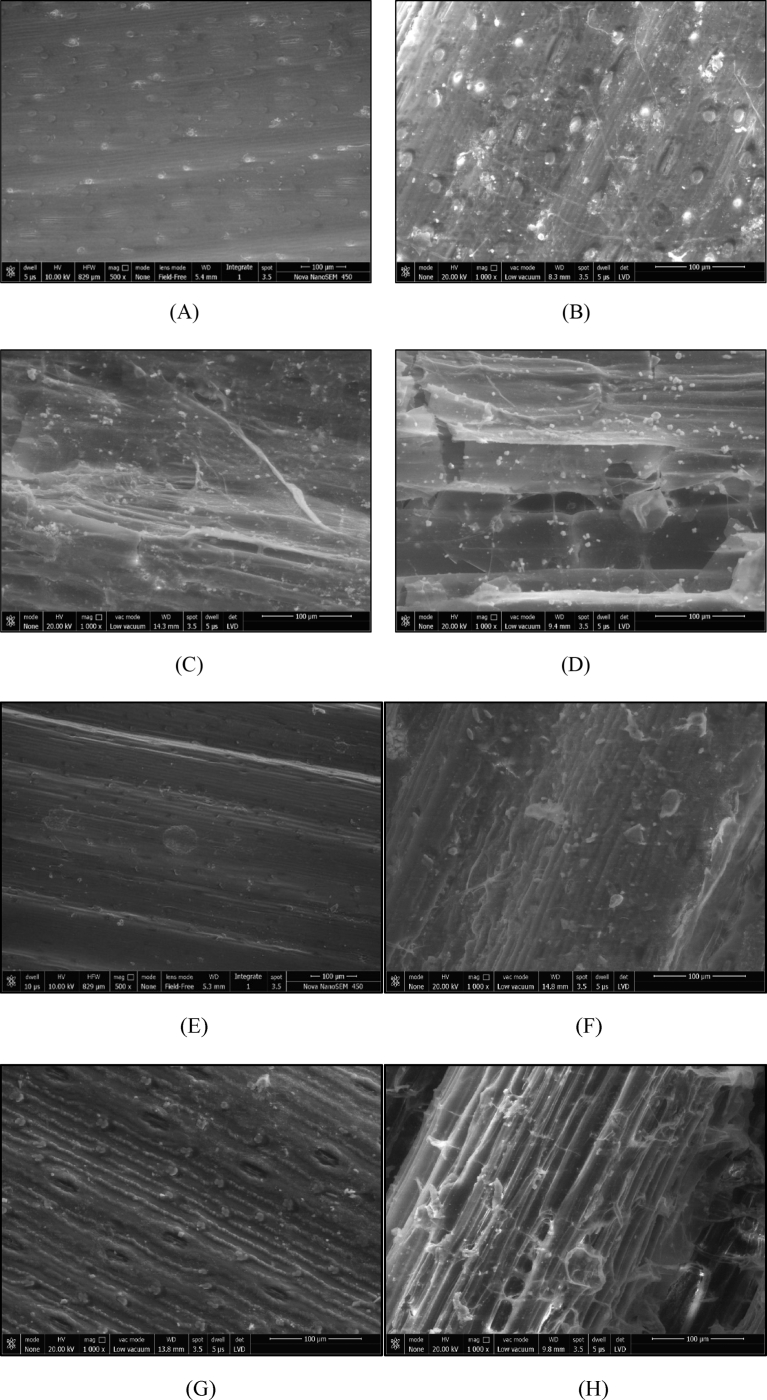
The SEM images of untreated and treated straw revealed the morphological changes occurred in the straw samples after treatment (Fig. 3). The characterization revealed the disruption on the surface of straw in treated samples which increased with the duration of treatment. As compared to the smooth and regular surface in untreated samples, the surface of treated samples was observed to be rough and irregular. Similar observations of structural deformations were reported by Karp et al. [15] after alkali pretreatment of corn stover with NaOH. Ramarajan and Manohar [16] also reported disrupted and worn out surfaces after biological pretreatment with Chaetomium globosum and Chaetomium brasiliense. Yadav et al. [17] reported similar observations after pretreatment of wheat straw and pearl millet straw with Pleurotus ostreatus.
Fig. 3.
SEM images of (A) untreated wheat straw (WS) (B) WS after 14 days pretreatment (C) WS after 28 days pretreatment (D) WS after 42 days pretreatment (E) untreated pearl millet straw (PMS) (F) PMS after 14 days pretreatment (G) PMS after 28 days pretreatment (H) PMS after 42 days pretreatment.
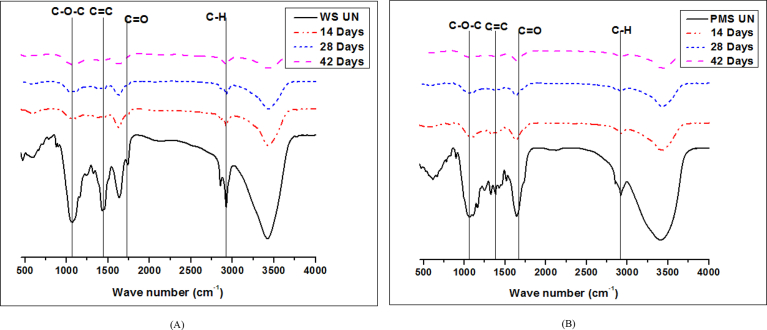
The FTIR spectra revealed the changes in the functional groups present in the samples (Fig. 4). The intensity of peak between 1400-1650 cm−1 which represents the C=C bonding was observed to be diminished in the treated samples thereby elaborating the breaking of C=C bonds found in the aromatic structural units of lignin [6, 18]. Earlier, Zheng et al. [19] reported the disappearance of peak between 1635-1655 cm−1 along with decrease in intensity of peaks at 1,620 and 1,460 cm−1 in the FTIR spectrum of alkali pretreated wheat straw. Yadav et al. [17] also reported decrease in intensity of peaks at 1420-1421 cm−1 after pretreatment of wheat and pearl millet straw with Pleurotus ostreatus depicting the breaking of aromatic structures in lignin due to lignolytic enzymes.
Fig. 4.
FTIR spectrum of (A) WS and (B) PMS.
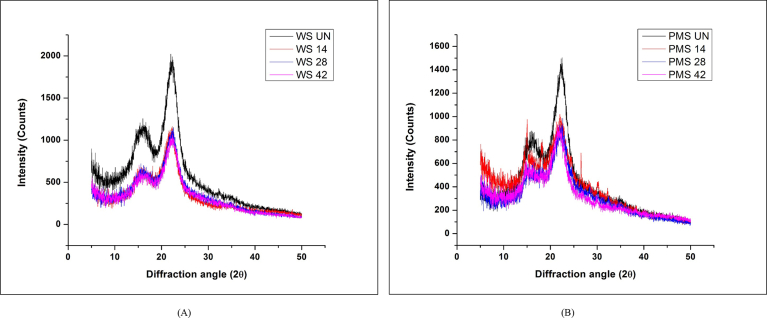
Furthermore, the impact of treatment on the crystallinity of straw samples was analyzed by determining the % Cr and C.I. through XRD. The XRD graphs showed the intensity of peaks for crystalline and amorphous cellulose in the untreated and treated straw samples (Fig. 5). Both the peaks for crystalline and amorphous contents were observed to be decline in the treated samples which revealed that along with lignin and hemicellulose (amorphous compounds), cellulose (crystalline compound) was also degraded during mycological treatment. The calculated % Cr and C.I. were also observed to be decreased after treatment (Table 1). Earlier, Meehnian et al. [6] also reported the similar effect on crystallinity after mycological treatment of cotton stalks with Daedalea flavida and Phlebia radiata. However, the trend of decrease in C.I. after mycological treatment was contrary to the earlier reports of chemical treatments where the C.I. was observed to decrease after enhancing the severity of treatment [20, 21]. It should be noticed that the chemical treatments specifically results into the degradation of the amorphous lignin, leaving the crystalline cellulose intact. This enhance the percentage of crystalline component in the samples, thereby enhancing the % Cr and C.I [20, 21]. However, in case of mycological treatment, the breakdown of lignin barrier enhanced the accessibility of cellulose to cellulase enzyme which resulted in degradation of the regular and compact arrangement of cellulose fibers also (as can be witnessed in the SEM images provided in Fig. 3). The degradation of cellulose and disruption of the fibers arrangement thereby resulted in the decrease in the C.I. The decrease in the C.I. observed during the mycological treatment is a positive outcome for preparing the straw for AD; since it is more convenient for the hydrolytic enzymes to find access through a less crystalline structure [22].
Fig. 5.
X-Ray diffraction spectrums for untreated and pretreated straw (A) WS; (B) PMS.
Table 1.
Impact of pretreatment on crystallinity of straw samples.
| S. No | Biomass | % Cr | C.I. (%) |
|---|---|---|---|
| 1 | Untreated WS | 67.6 | 52.2 |
| 2 | Treated WS (14 d) | 66.5 | 49.6 |
| 3 | Treated WS (28 d) | 66.1 | 48.8 |
| 4 | Treated WS (42 d) | 65.9 | 48.3 |
| 5 | Untreated PMS | 67.3 | 51.6 |
| 6 | Treated PMS (14 d) | 61.6 | 37.7 |
| 7 | Treated PMS (28 d) | 62.4 | 39.8 |
| 8 | Treated PMS (42 d) | 61.9 | 38.6 |
Here, WS: wheat straw; PMS: pearl millet straw.
3.4. Chemical composition analysis
The chemical composition analysis revealed the changes in the composition of lignin, cellulose and hemicellulose after mycological treatment (Table 2). Maximum lignin removal of 43% in wheat straw was observed after 42 days of treatment. In case of pearl millet straw, the maximum lignin removal of 41% was achieved after 42 days. The observed removal of lignin was comparable to the earlier reports of mycological treatment of lignocellulosic biomass. Saha et al. [23] achieved 46 and 51% degradation of lignin in corn stover after treatment with Cyathus stercoreus and Pycnoporus sanguineus respectively. On the other hand, Mustafa et al. [7] reported 23.3% removal of lignin after treatment of rice straw with Pleurotus ostreatus for 30 days. This is comparable to the 22% lignin removal obtained after 28 days treatment of pearl millet straw in the present study. Furthermore, Rastogi et al. [24] reported lignin removal as higher as 63% after mycological treatment with Pyrenophora phaeocomes.
Table 2.
Compositional analysis of untreated and treated straw samples.
| S.No | Type of straw | Lignin % | Cellulose % | Hemicellulose % | Lignin removal (%) |
|---|---|---|---|---|---|
| 1 | Untreated WS | 17.4 | 38.4 | 29.1 | … |
| 2 | Treated WS (14 d) | 14.3 | 36.9 | 28.9 | 17 |
| 3 | Treated WS (28 d) | 10.8 | 35.8 | 28.6 | 37 |
| 4 | Treated WS (42 d) | 9.9 | 35.5 | 28.7 | 43 |
| 5 | Untreated PMS | 15.6 | 36.4 | 25.2 | … |
| 6 | Treated PMS (14 d) | 13.7 | 35.6 | 24.6 | 12 |
| 7 | Treated PMS (28 d) | 12.1 | 34.7 | 24.1 | 22 |
| 8 | Treated PMS (42 d) | 9.2 | 34.3 | 23.7 | 41 |
Here, WS: wheat straw; PMS: pearl millet straw.
4. Conclusions
The present study explored the potential of Chaetomium globosporum to degrade the recalcitrant layer of lignin in wheat and pearl millet straw which is considered as the bottleneck for the hydrolysis stage of AD. The fungus was observed to be efficient in removing the lignin content in both types of straw and enhancing the quantity of released reducing sugars after treatment. Even though, degradation of cellulose and hemicellulose was also observed during the chemical compositional analysis. However, it was insignificant in comparison to the degradation of lignin. The mycological treatment was also observed efficient in reducing the crystallanity of straw samples which may be beneficial for the accessibility of hydrolytic enzymes during the biogas production process.
Declarations
Author contribution statement
Monika Yadav: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Vivekanand Vivekanand: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Department of Biotechnology and Department of Science and Technology Ministry of Science and Technology, Government of India through Ramalingaswami Re-Entry fellowship granted to Vivekanand Vivekanand (No. 288BT/RLF/Reentry/04/2013) and SERB-DST (ECR/2016/000989).
Competing interest statement
The authors declare that there is no conflict of interests.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors would like to acknowledge Centre for Energy and Environment, Malaviya National Institute of Technology Jaipur for providing fellowship and infrastructure to MY for carrying out the research.
References
- 1.Yadav M., Paritosh K., Chawade A., Pareek N., Vivekanand V. Genetic engineering of energy crops to reduce recalcitrance and enhance biomass digestibility. Agriculture. 2018;8(6):76. [Google Scholar]
- 2.Kumar S., Paritosh K., Pareek N., Chawade A., Vivekanand V. De-construction of major Indian cereal crop residues through chemical pretreatment for improved biogas production: an overview. Renew. Sustain. Energy Rev. 2018;90:160–170. [Google Scholar]
- 3.Sindhu R., Binod P., Pandey A. Biological pretreatment of lignocellulosic biomass – an overview. Bioresour. Technol. 2016;199:76–82. doi: 10.1016/j.biortech.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Y., Shi J., Tu M., Cheng Y.S. Chapter one - principles and development of lignocellulosic biomass pretreatment for biofuels. Adv. Bioenergy. 2017;2:1–68. [Google Scholar]
- 5.Yan X., Wang Z., Zhang K., Si M., Liu M., Chai L., Liu X., Shi Y. Bacteria-enhanced dilute acid pretreatment of lignocellulosic biomass. Bioresour. Technol. 2017;245 A:419–425. doi: 10.1016/j.biortech.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 6.Meehnian H., Jana A.K., Jana M.M. Pretreatment of cotton stalks by synergistic interaction of Daedalea flavida and Phlebia radiata in co-culture for improvement in delignification and saccharification. Int. Biodeterior. Biodegrad. 2017;117:68–77. [Google Scholar]
- 7.Mustafa A.M., Poulsen T.G., Sheng K. Fungal pretreatment of rice straw with Pleurotus ostreatus and Trichoderma resei to enhance methane production under solid-state anaerobic digestion. Appl. Energy. 2016;180:661–671. [Google Scholar]
- 8.Rouches E., Gimbert I.H., Steyer J.P., Carrere H. Improvement of anaerobic degradation by white-rot fungi pretreatment of lignocellulosic biomass: a review. Renew. Sustain. Energy Rev. 2016;59:179–198. [Google Scholar]
- 9.Xu G.B., Wang N.N., Bao J.K., Yang T., Li G.Y. New orsellinic acid esters from fungus Chaetomium globosporum. Helv. Chim. Acta. 2014;97:151–159. [Google Scholar]
- 10.Yadav M., Singh A., Balan V., Pareek N., Vivekanand V. Biological treatment of lignocellulosic biomass by Chaetomium globosporum: process derivation and improved biogas production. Int. J. Biol. Macromol. 2019;128:176–183. doi: 10.1016/j.ijbiomac.2019.01.118. [DOI] [PubMed] [Google Scholar]
- 11.Banakar S.P., Thippeswamy B. Isolation and partial purification of fungal ligninolytic enzymes from the forest soil fungi isolated from Bhadra Wildlife Sanctuary. Front. Biol. 2014;9(4):291–299. https://link.springer.com/article/10.1007/s11515-014-1319-x [Google Scholar]
- 12.Monssef R.A.A., Hassan E.A., Ramadan E.M. Production of laccase enzyme for their potential application to decolorize fungal pigments on aging paper and parchment. Ann. Agric. Sci. 2016;61(1):145–154. [Google Scholar]
- 13.Miller G.L. Use of dinitro salicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–432. [Google Scholar]
- 14.Harper S.H.T., Lynch J.M. The chemical components and decomposition of wheat straw leaves, internodes and nodes. J. Sci. Food Agric. 1981;32:1057–1062. [Google Scholar]
- 15.Karp E.M., Donohoe B.S., O’Brien M.H., Ciesielski P.N., Mittal A., Biddy M.J., Beckham G.T. Alkaline pretreatment of corn stover: bench-scale fractionation and stream characterization. ACS Sustain. Chem. Eng. 2014;2:1481–1491. [Google Scholar]
- 16.Ramarajan R., Manohar C.S. Biological pretreatment and bioconversion of agricultural wastes, using ligninolytic and cellulolytic fungal consortia. Ann. Finance. 2017;21(2):89–99. [Google Scholar]
- 17.Yadav M., Paritosh K., Pareek N., Vivekanand V. Coupled treatment of lignocellulosic agricultural residues for augmented biomethanation. J. Clean. Prod. 2019;213:75–88. [Google Scholar]
- 18.Singh D., Zeng J., Laskar D.D., Deobald L., Hiscox W.C., Chen S. Investigation of wheat straw biodegradation by Phanerochaete chrysosporium. Biomass Bioenergy. 2011;35:1030–1040. [Google Scholar]
- 19.Zheng Q., Zhou T., Wang Y., Cao X., Wu S., Zhao M., Wang H., Xu M., Zheng B., Zheng J., Guan X. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018;8(1):1321. doi: 10.1038/s41598-018-19517-5. https://www.nature.com/articles/s41598-018-19517-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akhtar N., Goyal D., Goyal A. Characterization of microwave-alkali-acid pre-treated rice straw for optimization of ethanol production via simultaneous saccharification and fermentation (SSF) Energy Convers. Manag. 2017;141:133–144. [Google Scholar]
- 21.Phitsuwan P., Permsriburasuk C., Baramee S., Teeravivattanakit T., Ratanakhanokchai K. Structural analysis of alkaline pretreated rice straw for ethanol production. Int. J. Polym. Sci. 2017 [Google Scholar]
- 22.Monlau F., Barakat A., Trably E., Dumas C., Steyer J.P., Carrere H. Lignocellulosic materials into biohydrogen and biomethane: impact of structural features and pretreatment. Crit. Rev. Environ. Sci. Technol. 2012;46:12217–12225. doi: 10.1021/es303132t. [DOI] [PubMed] [Google Scholar]
- 23.Saha B.C., Qureshi N., Kennedy G.J., Cotta M.A. Biological pretreatment of corn stover with white-rot fungus for improved enzymatic hydrolysis. Int. Biodeterior. Biodegrad. 2016;109:29–35. [Google Scholar]
- 24.Rastogi S., Soni R., Kaur J., Soni S.K. Unravelling the capability of Pyrenophora phaeocomes S-1 for the production of ligno-hemicellulolytic enzyme cocktail and simultaneous bio-delignification of rice straw for enhanced enzymatic saccharification. Bioresour. Technol. 2016;222:458–469. doi: 10.1016/j.biortech.2016.10.012. [DOI] [PubMed] [Google Scholar]