Figure 2.
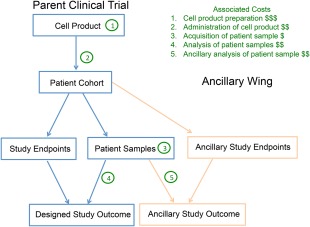
The relationship between parent and ancillary clinical trial highlighting shared costs. Parent clinical trial represents the primary focus of the clinical study. Cell products are administered to patients, patient samples are harvested for analysis, and various predetermined study endpoints are measured. Relative costs are indicated in green. The ancillary wing of the study would require the addition of ancillary study endpoints (different from parent trial, i.e., bone mineral density), and access to patient samples obtained from the parent trial for the ancillary analysis. The ancillary study costs (cost 5) would be minimal compared to the cost of the parent trial (costs 1–4).
