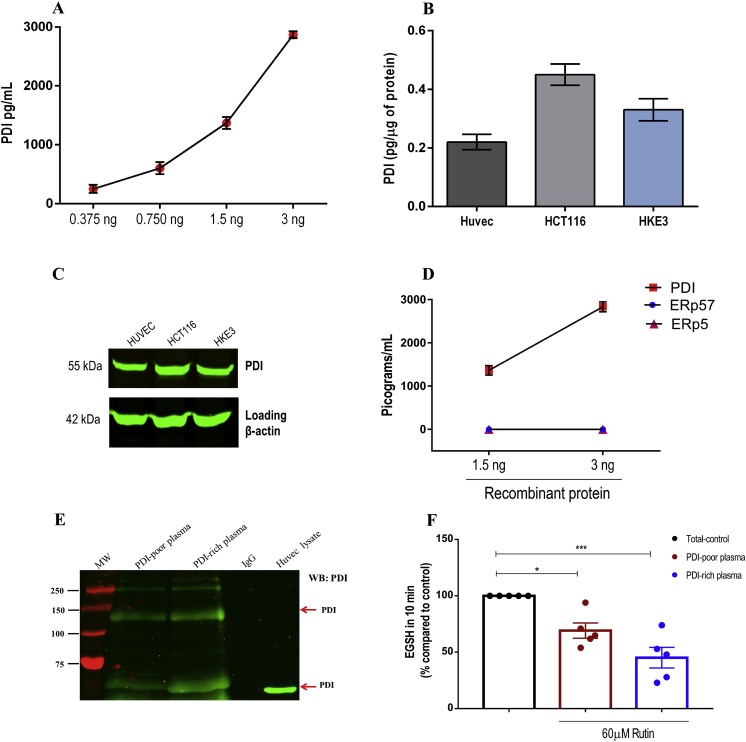
Fig. 1.
Validation of ELISA assay for specific detection of PDI. A. PDI concentration curves (0.375–3 ng/mL) were generated using purified PDI quantified by ELISA. Measurements were analyzed on 4 independent assays. Data represent mean ± SEM. B-C. Human cell lysates (HUVEC, HCT116 and HKE3) were (B) quantified by ELISA (n = 4; mean ± SEM) and (C) submitted to protein separation by reducing SDS-PAGE and immunoblotted with anti-PDI ELISA's capture antibody or anti-β actin (loading control) (n = 3). Uncropped western blots are shown in Supplementary Fig. S6. D. Cross-reactivity with other PDI family members was analyzed using recombinant PDI, ERp57 and ERp5 (1.5–3 ng/mL) quantified by ELISA. Measurements were analyzed on 4 independent assays. Data represent mean ± SEM. E. Plasma PDI immunoprecipitation. Platelet-poor plasma was diluted in lysis buffer and immunoprecipitated using rabbit anti-PDI antibody. Immunoblotting was performed using mouse anti-PDI (RL90) (n = 3 from independent experiments). F. Detection of plasma PDI reductase activity (di-eosin-GSSG assay) by formation of the reduced fluorogenic product eosin-5-isothiocyanate-coupled reduced glutathione (EGSH). It was measured in platelet-poor plasma exposed or not to 60 μM of rutin. Bar graphs represent the percentage cleavage of di-eosin-GSSG as compared to total reductase activity – Control (100%) in 10 min. The main result in this case is the rutin-inhibitable fraction of di-eosin-GSSG reductase activity. Data represent mean ± SEM from 5 independent experiments. *p < 0.05; ***p < 0.001 vs. control (One-way ANOVA followed by Tukey's post test).