Figure 3.
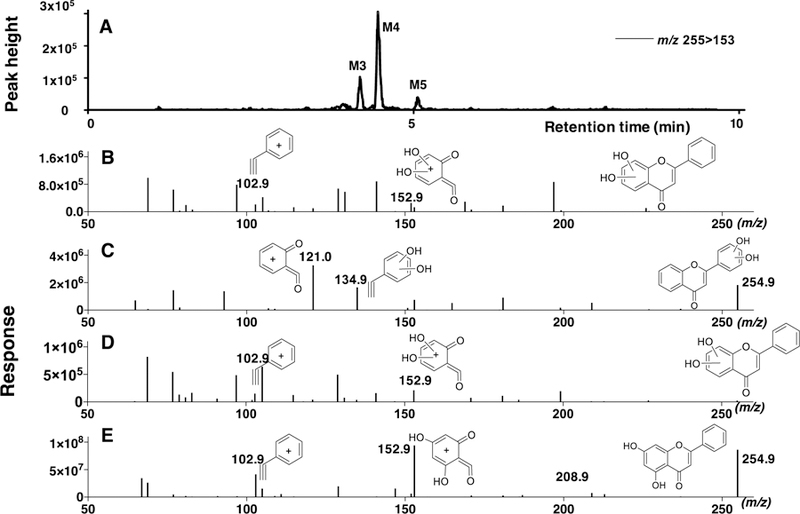
Metabolism of flavone by CYP2A6 to form di-hydroxylated flavones. LC-MS chromatograms of formation of di-hydroxylated metabolites (diOHF M3, diOHF M4, and diOHF M5) detected using the transitions m/z 255>153 are shown in Figure 3A. The reconstructed mass chromatograms of di-hydroxylated metabolites of diOHF M3 (B), diOHF M4 (C), and diOHF M5 (D) and of standard 5,7diOHF (E) are indicated in the lower part of the figure. The suggested structures of fragments of these metabolites are included in the figures.
