Figure 4. Combination of EPA with EPHA2 inhibition modifies plasma cell membrane lipid composition, polarity and apoptosis induction via ABCA1 inhibition in TNBC cell lines.
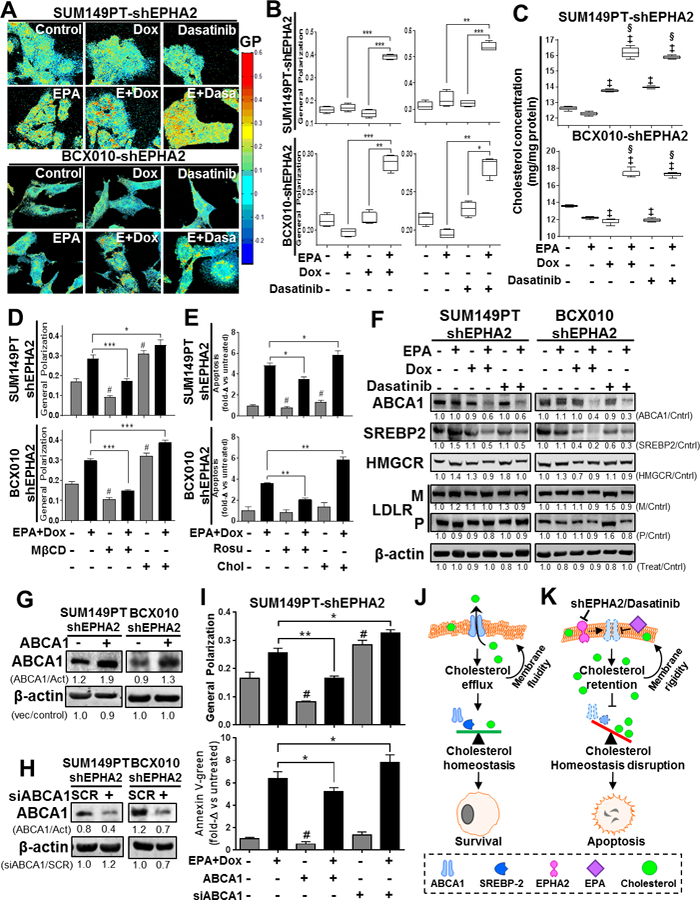
The effects of EPA in combination with EPHA2 inhibition on TNBC cell membranes were determined. (A-B) Cell membrane general polarization (GP, an indicator of polarity or rigidity) was determined in doxycycline (Dox)-inducible SUM149PT-shEPHA2 and BCX010-shEPHA2 cells treated with the indicated combinations of Dox, EPHA2 inhibitor dasatinib, and/or EPA. GP was determined by imaging (A) and quantification (B). Higher GP values indicate greater cell membrane polarization/rigidity. (C) Cholesterol concentrations in the membrane fractions of Dox-inducible SUM149PT-shEPHA2 and BCX010-shEPHA2 cells treated with indicated combinations of Dox, dasatinib, and/or EPA were determined by cholesterol quantification assay. (D-E) Dox-inducible SUM149PT-shEPHA2 and BCX010-shEPHA2 cells were treated with combination Dox and EPA as indicated, with or without cellular cholesterol removal (by methyl-β-cyclodextrin [MβCD] or rosuvastatin [Rosu]) or supplementation (Chol), and general polarization (D) and apoptosis (E) were quantified. Higher general polarization values indicate greater cell membrane polarization/rigidity. (F) Dox-inducible shEPHA2 cells were treated with EPA, Dox, dasatinib, or combinations and subjected to immunoblotting analysis of key cholesterol homeostasis-regulating proteins: ABCA1, mature SREBP2 (55–65 kDa), HMG CoA reductase (HMGCR), and LDL-receptor (LDLR) mature (M) and precursor (P) forms. (G-H) Inducible shEPHA2 cells were transfected with either an ABCA1-expressing (+) or empty control vector (−) (G) or pooled ABCA1 (+) or scrambled (SCR) control siRNA (H) and confirmed by immunoblotting assay. Pixel densities of individual proteins were quantified for each condition, and ratios of protein/β-actin normalized to control, or treated/untreated-control, are shown below the blots. β-actin expression was used as a protein loading control. Act, β-actin; Cntrl, control. (I) Dox-inducible shEPHA2 SUM149PT cells treated with combination EPA and Dox were transfected with ABCA1 expression vector or ABCA1 siRNA (siABCA1). General polarization of the cell membranes (top) and apoptosis (bottom) were quantified relative to untreated controls. Data were pooled from three independent experiments and are presented as mean ± SD, or mean of fold-change (fold-Δ) compared to untreated control ± SD. For all graphs, differences between groups were compared by unpaired t-test: *, P < 0.05; **, P < 0.001; *** P < 0.0001; ‡, P < 0.05 compared to EPA; §, P < 0.0001, compared to dasatinib or Dox; #, P < 0.05 compared to untreated control. (J-K) These schematics show the proposed mechanism of action for apoptosis induced by the combination of EPA and EPHA2-inhibiting therapy, involving increased cholesterol concentration and membrane polarization mediated by ABCA1 inhibition. (J) Cellular cholesterol homeostasis is normally maintained by the balancing effects of the cholesterol efflux channel protein ABCA1 and cholesterol biosynthesis inducer SREBP2. Their activity promotes maintenance of plasma cell membrane polarity and cellular survival. (K) Treatment with combination of EPA with EPHA2 inhibition (shEPHA2 or dasatinib) alters the cell membrane structure, inhibiting the cholesterol-exporting functions of ABCA1. This results in the cellular accumulation of cholesterol and subsequent increase in cell membrane polarity. This aberrant accumulation of intracellular/membrane cholesterol prevents the activity of cholesterol biosynthesis inducer proteins (e.g., SREPB2) and disrupts cellular cholesterol homeostasis, resulting in apoptosis.
