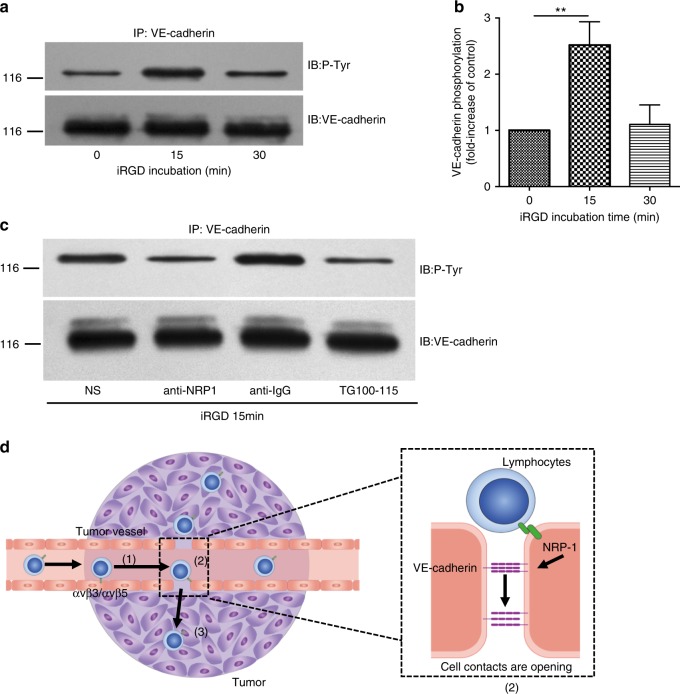
Fig. 4.
Potential mechanism of the effects exerted by iRGD on the extravasation of lymphocytes in tumors. a iRGD induced tyrosine phosphorylation of VE-cadherin in endothelial cells. Endothelial cells were incubated with iRGD and subjected to immunoprecipitation of VE-cadherin at the indicated time. The immunoprecipitate was then analyzed by immunoblotting using either anti-phospho-tyrosine or anti-VE-cadherin antibody. b The amount of tyrosine-phosphorylated VE-cadherin in panel (a) was quantified by densitometry from three independent experiments and expressed as fold-increase of untreated controls. Data represent mean ± s.e.m.; Student’s t test. ***p < 0.001. c Anti-NRP-1 antibody and PI3Kγ/δ inhibitor TG100–115 blocked iRGD-induced tyrosine phosphorylation of VE-cadherin. Endothelial cells were incubated with 15 μg ml−1 anti-NRP-1 antibody, control sheep anti-IgG or 3.5 μg ml−1 TG100-115 prior to iRGD incubation, tyrosine phosphorylation of VE-cadherin was analyzed by immunoprecipitation. d Schematic diagram of iRGD working mechanism. Circulating iRGD-modified T cells tether and roll in blood flow via the engagement of αvβ3/αvβ5 expressed on tumor vascular endothelial cell, slowing its velocity (1). The interaction also initiates the proteolysis of iRGD and expose the CendR motif. The truncated peptide then bind to NRP-1, triggering the tyrosine phosphorylation of VE-cadherin and the formation of intercellular gaps (2).The connected lymphocytes then cross the vessel wall and infiltrate into tumor parenchyma (3)