Abstract
Background
Measurement of serum creatinine (sCr) and urea nitrogen fail to detect decreased renal function in many hyperthyroid cats because of low muscle mass and glomerular hyperfiltration of affected cats. Serum symmetric dimethylarginine (sSDMA) is an earlier and more sensitive renal biomarker than sCr.
Objective
Evaluate sSDMA as a biomarker of renal function in hyperthyroid cats before (T0) and 1 month after (T1) radioiodine (131I) treatment.
Animals
Forty‐seven client‐owned hyperthyroid nonazotemic cats were evaluated at T0 and T1.
Methods
A prospective study in which sCr and sSDMA concentrations were determined in 47 hyperthyroid cats at T0 and at T1. Glomerular filtration rate (GFR) was estimated at T0 and T1 in 10 of these 47 cats using plasma exogenous creatinine clearance test.
Results
Serum SDMA was elevated (>14 μg/dL) in 6 of 47 cats at T0 and normalized after treatment in 4 of those cats. All cats remained nonazotemic after treatment. In 10 cats in which GFR was measured, correlation between GFR and sSDMA was low and not significant (τb = −0.35, P = .17 at T0 and τb = −.22, P = .41 at T1), whereas correlation between GFR and sCr was moderate and significant (τb = −0.52, P < .05 at T0 and τb = −.53, P = <.05 at T1).
Conclusions and Clinical Importance
Careful interpretation of mildly increased sSDMA with normal sCr in hyperthyroid cats is warranted as sSDMA values might normalize after resolution of hyperthyroidism in some cats. In this population of hyperthyroid cats, sSDMA was poorly correlated with GFR.
Keywords: 131I, chronic kidney disease, CKD, feline, GFR, glomerular filtration rate, hyperthyroidism, symmetric dimethylarginine
Abbreviations
- 131I
radioiodine
- BCS
body condition score
- BP
blood pressure
- BW
body weight
- CKD
chronic kidney disease
- GFR
glomerular filtration rate
- LC‐MS
liquid chromatography‐mass spectroscopy
- MCS
muscle condition score
- RI
reference interval
- sCr
serum creatinine
- SDMA
symmetric dimethylarginine
- sSDMA
serum symmetric dimethylarginine
- T0
pre‐treatment time point
- T1
1 month post‐treatment time point
- TSH
thyroid‐stimulating hormone
- TT4
total thyroxine
- UPC
urinary protein : creatinine ratio
- USG
urine specific gravity
1. INTRODUCTION
Hyperthyroidism and chronic kidney disease (CKD) are common disorders in aged cats and frequently occur together. Glomerular hyperfiltration and loss of muscle mass in cats with hyperthyroidism often lead to decreased serum creatinine (sCr) which can hamper the diagnosis of CKD in these patients. Azotemia is observed in 10% of hyperthyroid cats at diagnosis, and 15%‐60% cats develop renal azotemia after treatment.1, 2, 3, 4 Early identification of decreased renal function in hyperthyroid cats could contribute to the best treatment choice of hyperthyroidism and allow earlier management of CKD. Closer follow‐up of radioiodine (131I)‐treated cats with early CKD could prevent potential long‐standing iatrogenic hypothyroidism which can contribute to exacerbation of azotemia and reduced survival time.5
Routinely used renal biomarkers, sCr and serum urea, are neither sensitive nor specific biomarkers to detect renal dysfunction. The influence of muscle mass and analytic variability of sCr leads to its poor sensitivity.6, 7 Glomerular filtration rate (GFR) is considered the gold standard to evaluate renal function but is usually described as time demanding and stressful for cats.8 Glomerular filtration rate limited sampling strategies were developed to diminish these difficulties.9 Despite that, GFR is still not routinely used in clinical settings. There is a need for early renal markers not influenced by nonrenal factors, especially in often poorly muscled hyperthyroid cats.
Symmetric dimethylarginine (SDMA) is a byproduct of protein methylation, is minimally protein bound, and is freely filtered through the glomerulus. In people, more than 90% of SDMA is eliminated by renal clearance.10 Symmetric dimethylarginine closely correlates with GFR in healthy elderly cats and cats with CKD.11, 12, 13 Sensitivity and specificity of SDMA to identify decreased renal function in cats with CKD is 100% and 91%, respectively.13 Furthermore, in healthy Beagles, a reduction of lean body mass can lower sCr concentration and therefore overestimate GFR, whereas SDMA is not influenced by lean body mass changes.14 There is a positive correlation of sCr with lean body mass but no correlation of SDMA with lean body mass in healthy elderly cats suggesting that sCr concentration is more affected by age and lean body mass than SDMA.12 Based on these characteristics, SDMA is expected to be a better renal marker than sCr in hyperthyroid cats. Symmetric dimethylarginine has higher sensitivity to predict renal azotemia than sCr in hyperthyroid cats although sensitivity of SDMA is as low as 33%.15 Further evaluation of SDMA in hyperthyroid cats is needed.
The objective of this study was to evaluate SDMA as a renal biomarker in hyperthyroid cats along with GFR and sCr before and 1 month after 131I treatment.
2. MATERIALS AND METHODS
2.1. Animals
Fifty client‐owned hyperthyroid cats treated with 131I at the Small Animal Department of Ghent University were prospectively enrolled in the study. Approval from the Local Ethical Committee of Ghent University (EC 2015/67) was obtained, and owners signed an informed consent. Inclusion criteria were diagnosis of hyperthyroidism based on clinical signs, increased serum total thyroxine (TT4) concentration, and increased pertechnetate uptake in one or both thyroid glands or ectopic thyroid tissue on scintigraphic scan. Exclusion criteria were azotemia (sCr > 2.3 mg/dL, reference interval [RI] of IDEXX Laboratories, Inc, Westbrook, Maine), any clinically relevant systemic disease other than hyperthyroidism, and use of any medication within 2 weeks before enrolling in the study except for antithyroid medication. Antithyroid medication was stopped 10 days before 131I treatment. Cats had to have a consistent diet at least 1 month before and during the study. Cats on raw meat diet were excluded. Cats were treated with an individually adapted dose of 131I injected IV (mean dose 142.6 MBq; range: 67.7‐455.1 MBq). The dose was based on the severity of the clinical signs, serum TT4, and thyroid‐to‐salivary gland ratio as determined on the pertechnetate scan.16
2.2. Sampling
Cats were evaluated shortly (4‐48 hours) before (T0) and 1 month after (T1) 131I treatment. Full health screening was performed at T0 including medical history, physical examination, systolic blood pressure (BP), CBC, biochemistry profile, urinalysis, urine culture, abdominal ultrasonography, standard 2‐view thoracic radiographs (lateral and ventrodorsal projections), and echocardiography.17 At both time points (T0 and T1), body weight (BW), body condition score (BCS) (9‐point scale), muscle condition score (MCS), BP, serum TT4, serum thyroid‐stimulating hormone (TSH), sCr, serum urea, sSDMA, GFR, urine specific gravity (USG), and urinary protein : creatinine ratio (UPC) were assessed. A noninvasive BP was measured using Doppler ultrasonic technique following the ACVIM consensus.18 All blood samples were centrifuged within 30 minutes after collection, serum separated and stored at 4°C until sent to commercial laboratory within 24 hours from collection. Samples for TSH were stored at −80°C and sent for batch analysis at the end of the study. Serum creatinine was determined by an enzymatic colorimetric method, Jaffé's reaction using picrate at alkaline pH (Beckman Coulter, Inc, Brea, California), and azotemia was defined as sCr > 2.3 mg/dL based on upper limit of sCr RI established by IDEXX Laboratories. Serum SDMA was determined using a commercially available high‐throughput immunoassay (IDEXX SDMA Test), and sSDMA RI ≤ 14 μg/dL) was established by IDEXX Laboratories.19 Serum TT4 concentration was measured on an automated biochemistry analyzer using a homogenous enzyme immunoassay (DRI T4 assay; Microgenics Corporation, Freemont, California). Serum TSH was determined using canine TSH chemiluminescent enzyme immunoassay (Immulite Canine TSH; Siemens Healthcare Diagnostics Products, Tarrytown, New York).
2.3. Glomerular filtration rate
Exogenous plasma creatinine clearance testing was offered to owners and performed in 10 of 47 cats at T0 and T1 using protocol previously established by our research group.20 The number of blood samplings in the protocol was adapted based on a published limited sampling strategy for research purposes.9 More specifically, catheter was placed in the cephalic vein, baseline blood sample was obtained, and 40 mg/kg of creatinine were injected IV. Blood samples were collected into an EDTA tube 5, 30, 60, 120, 180, 360, and 600 minutes after creatinine injection. All samples were centrifuged within 1 hour from collection, and separated plasma was stored at 4°C until sent to the commercial laboratory the next day. Plasma creatinine was determined using enzymatic colorimetry as described above. Glomerular filtration rate was estimated using a noncompartmental approach with extrapolation to infinity. The clearance was equal to the dose divided by the area of the concentration versus time curve. The borderline low GFR cutoff value was defined as 1.9 mL/min/kg and the low GFR cutoff value as 1.4 mL/min/kg based on previous data from our research group.9
2.4. Thyroid status
One month after treatment with 131I, cats were classified as euthyroid if serum TT4 concentration was normal or below RI (0.8‐4.7 μg/dL) with normal serum TSH concentration (≤0.3 ng/mL), as hypothyroid if serum TT4 concentration was below RI with increased serum TSH concentration (>0.3 ng/mL), or as subclinically hypothyroid if serum TT4 concentration was normal with increased serum TSH concentration (>0.3 ng/mL), and as hyperthyroid if serum TT4 was above RI with TSH <0.3 ng/mL.5, 21
2.5. Statistical analysis
The effect of the treatment was assessed by the signed rank test with cat as blocking factor, as not all response variables could be assumed to be normally distributed. Kendall's τ correlation coefficients were derived to investigate the correlation between different response variables. With 47 cats, the power for a paired test to prove a difference in SDMA between T0 and T1 at the 5% significance level, assuming an SD equal to 4.8 and a true difference of 2 is equal to 80%.
3. RESULTS
3.1. Animals
Fifty client‐owned hyperthyroid cats treated with 131I were enrolled in the study. Three cats were excluded because of owner noncompliance (n = 1), congestive heart failure (n = 1), and thyroid carcinoma with pulmonary metastasis (n = 1). Of the 47 included cats, 22 were male neutered, 24 female spayed, and 1 female intact. Represented breeds included 44 domestic short‐hair or long‐hair cats, 1 British Shorthair, 1 Norwegian Forest Cat, and 1 Chartreux. Median age was 13 years (range 7‐16 years). Selected clinical and laboratory variables of the total group are summarized in Table 1. Twenty‐one of 47 (45%) patients were previously treated for hyperthyroidism with methimazole (Felimazole; Dechtra Veterinary Products, Overland Park, United Kingdom) (n = 17), with carbimazole (Teva, Haarlem, the Netherlands) (n = 1), with combination of unilateral thyroidectomy and methimazole (Dechtra Veterinary Products) (n = 1) or with Hill's Y/D diet (Hill's Pet Nutrition, Inc, Topeka, Kansas) (n = 2). Body weight and BCS significantly increased after treatment (P < .001). Muscle condition score overall significantly improved after treatment (P < .001); MCS improved in 28 cats (60%), remained stable in 17 cats (36%), and worsened in 2 cats (4%) after treatment. The 1 cat that had persistent severe muscle loss remained hyperthyroid after treatment.
Table 1.
Selected clinical and laboratory variables of total group (n = 47) before (T0) and 1 month after (T1) radioiodine treatment provided as median (range)
| Variables (RI + units) | T0 | T1 | Median of difference between T1 and T0 (range) | P |
|---|---|---|---|---|
| Age (years) | 13 (7–16) | |||
| BW (kg) | 3.8 (1.5‐6.5) | 4.2 (2.0‐6.4) | 0.2 (−0.3 to 1.1) | <.001 |
| BCS | 4/9 (2‐8 /9) | 5/9 (2–8 /9) | 0 (−1 to 2) | <.001 |
| MCS | Normal; n = 7 (15%) | Normal; n = 22 (47%) | Improved | <.001 |
| Mild loss; n = 20 (43%) | Mild loss; n = 18 (38%) | |||
| Moderate loss; n = 15 (32%) | Moderate loss; n = 6 (13%) | |||
| Severe loss; n = 5 (10%) | Severe loss; n = 1 (2%) | |||
| BP (mm Hg) | 160 (125‐225) | 165 (125‐220) | 0 (−70 to 50) | .67 |
| Serum TT4 (0.8‐4.7 μg/dL) | 8.3 (2.2‐>13) | 0.9 (<0.7‐8) | −6.7 (<−12.3 to −0.6) | <.001 |
| Serum TSH (<0.3 ng/mL) | <0.03 (<0.03‐0.04) | 0.09 (<0.03‐0.95) | −0.05 (0 to 0.92) | <.001 |
| Serum creatinine (0.9‐2.3 mg/dL) | 0.67 (0.39‐1.55) | 1.23 (0.39‐2) | 0.52 (−0.57 to 1.17) | <.001 |
| Serum urea (16‐38 mg/dL) | 22.7 (12.6‐53.5) | 28.0 (17.4‐58.8) | 4.2 (−6.2 to 20) | <.001 |
| Serum SDMA (0‐14 μg/dL) | 10 (1‐18) | 11 (6‐20) | 0.0 (−7 to 19) | .61 |
| USG | >1.050 (1.016‐>1.050) | 1.042 (1.013‐>1.050) | −0.008 (−0.037 to 0.010) | <.001 |
| UPC (<0.33) | 0.5 (0.2‐2.4) | 0.2 (0.1‐0.82) | −0.3 (−2.1 to 0.12) | <.001 |
Abbreviations: BCS, body condition score; BP, blood pressure; BW, body weight; MCS, muscle condition score; RI, reference interval; SDMA, symmetric dimethylarginine; TSH, serum thyroid‐stimulating hormone; TT4, serum total thyroxine; UPC, urinary protein : creatinine ratio; USG, urine specific gravity.
3.2. Serum renal biomarkers, USG and UPC
Serum creatinine concentration significantly increased 1 month after 131I treatment compared to T0 (Table 1). Serum creatinine increased in 44 cats (94%), decreased in 2 cats (4%) and remained unchanged in 1 cat (2%) after treatment (Figure 1). Despite significant sCr increase 1 month after treatment, all 47 cats remained nonazotemic (sCr <2.3 mg/dL) during the study period. The serum urea concentration significantly increased after treatment. There was no significant difference between sSDMA at T0 and T1 (Table 1). Serum SDMA increased in 19 cats (40%), decreased in 23 cats (49%), and remained unchanged in 5 cats (11%) after 131I treatment compared to T0 (Figure 1). Serum SDMA was above RI in 6 cats at T0 and in 5 cats at T1. Serum SDMA normalized after 131I treatment in 4 of the 6 cats with increased concentrations before treatment. Serum SDMA exceeded 14 μg/dL at T1 in 3 cats that had normal sSDMA at T0. The correlation between sCr and sSDMA was low at both time points (τb = 0.31, P < .01 at T0 and τb = 0.29, P < .01 at T1). The USG and UPC significantly decreased after treatment. Urine specific gravity was <1.035 in 9 cats at T0 and in 20 cats at T1. Three of 6 cats with increased sSDMA at T0 had also USG < 1.035 and 2 of 4 cats in which sSDMA normalized after treatment had USG < 1.035. Four of 5 cats with increased sSDMA at T1 had also USG < 1.035.
Figure 1.
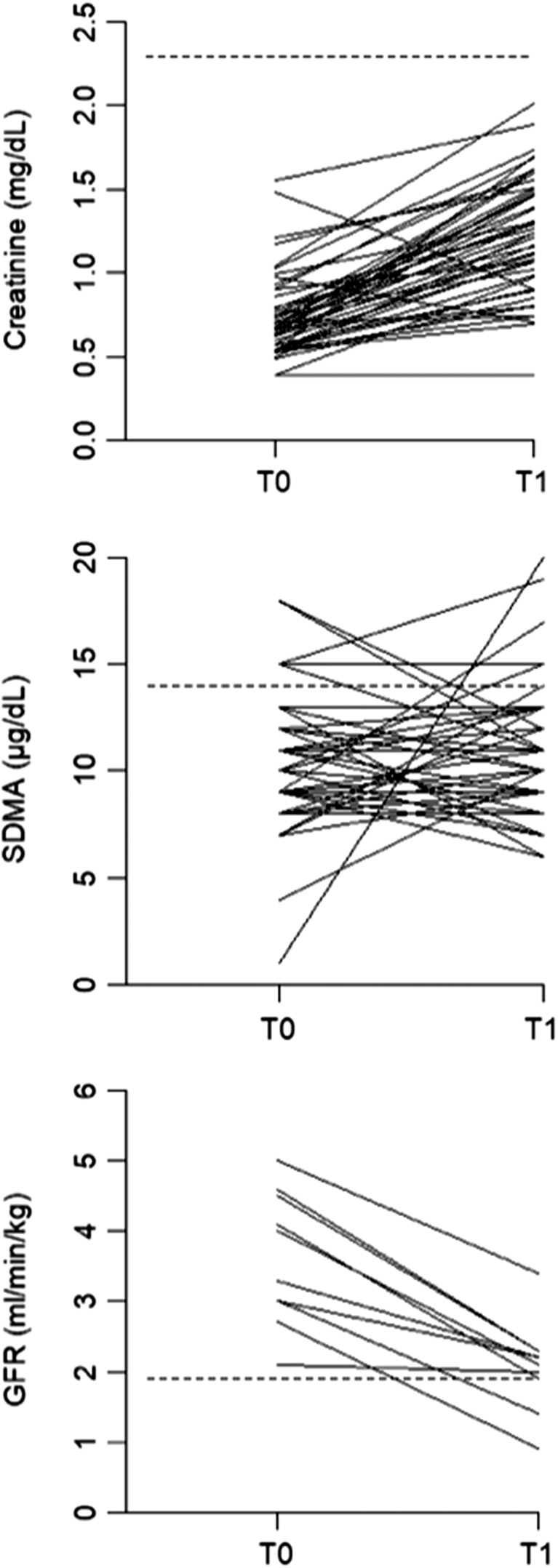
Serum creatinine, serum SDMA, and GFR before (T0) and 1 month after (T1) radioiodine treatment. Dotted lines show upper limit of reference interval for SDMA and creatinine and borderline low cutoff value for GFR. GFR, glomerular filtration rate; SDMA, symmetric dimethylarginine
3.3. Thyroid status
Total serum thyroxine was significantly lower and serum TSH was significantly higher at T1 compared to T0 (Table 1). One month after 131I treatment, 1 cat (2%) remained hyperthyroid, 41 cats (87.5%) were euthyroid, 3 cats (6.5%) were subclinically hypothyroid, and 1 cat (2%) was hypothyroid. Unknown thyroid status was assigned to 1 cat (2%) in which serum TSH concentrations was missing and TT4 concentration was below RI. All subclinically hypothyroid and hypothyroid cats had normal sCr and sSDMA at T0 and T1 and 2 cats had USG < 1.035.
Serum TT4 and creatinine showed moderate and significant negative correlation at T0 (τb = −0.39, P < .001) and low and not significant negative correlation at T1 (τb = 0.09, P = .4). Serum TT4 and SDMA showed low and not significant negative correlation at both time points (τb = −0.05, P = .61 at T0 and τb = −0.13, P = .26 at T1). Correlation between serum TSH and sCr was low and not significant at T0 but moderate and significant at T1 (τb = 0.1, P = .16 at T0 and τb = 0.52, P < .001 at T1). Correlation of serum TSH and sSDMA was low and not significant at both time points (τb = 0.04, P = .78 at T0 and τb = 0.15, P = .18 at T1).
There was no significant difference in sCr, sSDMA, serum urea, USG, or BW between the group of euthyroid cats and the group of subclinically hypothyroid and hypothyroid cats.
3.4. Glomerular filtration rate
Selected clinical and laboratory variables of the subgroup of cats with GFR are summarized in Table 2. Four of ten cats with GFR (40%) were pretreated with antithyroid medication compared to 45% pretreated cats in the total population. When the subgroup of cats with GFR was compared with the total population of cats for age, BW, sCr, sSDMA, TT4, and BP, no statistical significant difference was found at T0 and T1. Urine specific gravity was significantly higher in GFR subgroup compared to the total population at both time points.
Table 2.
Selected clinical and laboratory variables of subgroup of cats with GFR performed (n = 10) before (T0) and 1 month after (T1) radioiodine treatment provided as median (range)
| Variables (RI + units) | T0 | T1 | Median of difference between T1 and T0 | P |
|---|---|---|---|---|
| Age (years) | 12.5 (8‐15) | |||
| BW (kg) | 4.0 (2.6‐5.1) | 4.2 (2.9‐5.1) | 0.3 (0‐0.6) | <.001 |
| MCS | Normal; n = 2 (20%) | Normal; n = 7 (70%) | Improved | <.001 |
| Mild loss; n = 4 (40%) | Mild loss; n = 3 (30%) | |||
| Moderate loss; n = 3 (30%) | ||||
| Severe loss; n = 1 (10%) | ||||
| BP (mm Hg) | 163 (140‐225) | 170 (125‐200) | 5 (−10 to 50) | .67 |
| Serum TT4 (0.8‐4.7 μg/dL) | 10 (4.8‐>13) | 0.8 (<0.7‐1.3) | −8.9 (<−12.2 to −3.5) | <.001 |
| Serum creatinine (0.9‐2.3 mg/dL) | 0.66 (0.39‐1) | 1.21 (0.97‐1.5) | 0.54 (0.27 to 0.85) | <.001 |
| Serum SDMA (0–14 μg/dL) | 9.5 (4‐15) | 11 (7‐17) | 2.5 (−5 to 8) | .2 |
| USG | >1.050 (1.040‐>1.050) | >1.050 (1.032‐>1.050) | 0 (−0.008 to 0.010) | .42 |
| GFR (≥1.9 mL/min/kg) | 3.65 (2.1‐5) | 2.15 (0.9‐3.4) | −1.7 (−2.2 to −0.1) | .002 |
Abbreviations: BCS, body condition score; BP, blood pressure; BW, body weight; MCS, muscle condition score; RI, reference interval; SDMA, symmetric dimethylarginine; TSH, serum thyroid‐stimulating hormone; TT4, serum total thyroxine; UPC, urinary protein : creatinine ratio; USG, urine specific gravity.
Glomerular filtration rate was measured at T0 and T1 in 10 of 47 included cats. Glomerular filtration rate significantly decreased after treatment (Table 2; Figure 1). None of the cats had low or borderline low GFR before treatment. At T1, 1 cat had low GFR and 1 cat borderline low GFR. The cat with borderline low GFR was euthyroid and had increased sSDMA at T1. The cat with low GFR was euthyroid and had normal sSDMA at T1. Serum SDMA after treatment was normal, and USG was ≥1.035 at T0 in both these cats and USG was <1.035 at T1 in 1 of them. Correlation between GFR and sSDMA (n = 10) was low and not significant at both times (τb = −0.35, P = .17 at T0 and τb = −0.22, P = .41 at T1), whereas correlation between GFR and sCr was moderate and significant in both times (τb = −0.52, P < .05 at T0 and τb = −0.53, P = <.05 at T1).
4. DISCUSSION
This study evaluates SDMA in conjunction with sCr and GFR in hyperthyroid cats treated with 131I. We found a moderate correlation of sCr with GFR and a low correlation of sSDMA with GFR and with sCr. Serum SDMA did not significantly change after treatment which is in contrast to significant increase of sCr and significant decrease of GFR after treatment. Furthermore, mildly increased sSDMA before treatment normalized in some cats after 131I treatment. Although based on low numbers, sCr and sSDMA before treatment did not predict abnormally low GFR after treatment.
The correlations of sSDMA with GFR and sSDMA with sCr were low and not significant at both times (T0 and T1). This is in contrast to high and significant correlation of SDMA with GFR and SDMA with sCr in previous studies evaluating healthy elderly and CKD cats.11, 12, 13 Although GFR significantly decreased after treatment in all 10 cats, there was no obvious pattern of sSDMA changes in our complete study population (see Figure 1). This confirms the poor correlation of GFR with SDMA in our hyperthyroid cats. As expected, GFR and sCr correlation in our population was only moderate because sCr is known to be a poor renal marker in hyperthyroid cats. In a recent study, hyperthyroid cats managed with controlled iodine diet showed higher sSDMA correlation with GFR (r = 0.71, P = .04) than sCr with GFR (0.55, P = .04), which is in contrast with the present results. In that study, sCr significantly decreased and GFR did not change over a period of 6 months on controlled iodine diet; sSDMA also did not change over time. Interestingly, only 5 of 15 cats in that study achieved euthyroidism based on serum TT4 concentrations, which could explain why GFR did not decrease and sCr did not increase as expected after resolving hyperthyroidism.22
Data of subgroup of cats in which GFR (n = 10) was measured show that neither sCr nor sSDMA perform well as renal biomarkers in hyperthyroid cats. Glomerular hyperfiltration in hyperthyroidism leads to increased GFR which may influence also renal filtration of SDMA. This could explain why sSDMA before treatment did not predict low GFR after treatment in our study population. Serum SDMA after treatment was above RI in 1 of 2 cats with abnormally low GFR. These 2 cats were euthyroid after treatment and therefore hypothyroidism causing decreased GFR was excluded. Serum SDMA sensitivity (33.3%) to predict azotemia after treatment of hyperthyroidism in cats is higher than sCr sensitivity (11.9%).15 Nevertheless, these results indicate that SDMA is still a poor predictor of after treatment azotemia.
Serum SDMA was increased before treatment in 6 cats (13%) of our study population and normalized after treatment in 4 of them. Only 1 of these 6 cats had GFR performed which was normal in both times. Similarly in a recent study, SDMA normalized after 131I treatment in 3 of 5 nonazotemic cats with increased pretreatment SDMA.15 Furthermore, in a study of 19 methimazole treated hyperthyroid cats, SDMA normalized after starting methimazole in 1 of 2 cats in which follow‐up was available.23 Therefore, temporarily mildly increased serum SDMA should be interpreted carefully while evaluating pretreatment renal function in hyperthyroid cats. Close follow‐up of both renal markers (creatinine and SDMA) after treatment is recommended to avoid misinterpretation.
There is a positive association of SDMA with increased thyroid function in humans.24 Serum SDMA is a byproduct of protein methylation, and protein metabolism is considerably changed in hyperthyroid cat. Therefore, we can speculate that hyperthyroidism might influence SDMA concentration in cats. The production rate of SDMA in the body is relatively stable, but protein breakdown could potentially contribute to changes in serum concentration.7 Furthermore, SDMA is freely filtered by the kidneys, but there is also hepatic clearance in people.25 The extent of hepatic SDMA clearance and whether it changes in hyperthyroidism is unknown in cats. Nevertheless, there was no correlation between serum TT4 or serum TSH and SDMA in our study, which is in agreement with a recent study in hyperthyroid cats showing also very weak correlation of serum SDMA and TT4 concentrations.15
The present study showed expected evolution of sCr, serum urea, and GFR, where sCr and serum urea significantly increased and GFR significantly decreased after treatment of hyperthyroidism. Nevertheless, none of the cats became azotemic after treatment. This is in contrast with literature where 15%‐60% of hyperthyroid cats become azotemic after treatment.2, 3, 4 In our study, 45% of cats were previously treated with antithyroid medication or with controlled iodine diet which might have played a role in preselection of nonazotemic cats. Furthermore, the increase of creatinine might have been hampered by incomplete resolution of muscle mass loss 1 month after treatment in about a half of the cats. Longer follow‐up would be necessary to assess if some cats would develop azotemia later. On the other hand, our study design was based on previously published data of our research group showing that renal function stabilizes 4 weeks after 131I treatment and there is no significant decline in renal function later on.26 Based on the findings of that study, we should be able to recognize cats with impaired renal function 1 month after treatment based on sCr levels. This reasoning is further supported by another study showing that GFR significantly decreases 1 month after 131I treatment, but there is no further significant GFR decrease between 1 and 6 months after treatment.27 In that study, creatinine further increased between 1 and 6 months after treatment which was most likely a result of muscle wasting resolution or prerenal azotemia considering that GFR remained stable.27 Furthermore, long‐term follow‐up could show development of CKD unrelated to resolution of hyperthyroidism. Up to 30% of previously healthy geriatric cats develop azotemia during 12‐month follow‐up.28 This further supports that short‐term follow‐up should be suitable to evaluate kidney function after treatment of hyperthyroidism. A possible explanation for lack of azotemia after treatment in our study population might be the laboratory RI for sCr. Serum creatinine has a large biologic and analytic variability which often leads to overly wide RIs established by laboratories.7
Urinary protein : creatinine ratio significantly decreased after treatment; this is an expected evolution as glomerular hyperfiltration, changes in glomerular barrier, as well as changes in tubular protein management increase proteinuria in hyperthyroidism, and proteinuria decreases quickly after resolution of hyperthyroidism in people.1 Urine specific gravity significantly decreased after treatment, and more cats had USG <1.035 at T1 than at T0. Despite significant decrease of USG, the median value remained above 1.035 after treatment. Hyperthyroidism can lead to decreased ability to concentrate urine without clinical significance.1 We would expect that cats with normal kidney function would be able to concentrate urine after resolution of hyperthyroidism. The decrease of USG might reflect early kidney dysfunction or hypertension‐induced diuresis.28, 29, 30
As expected, BW and BCS significantly increased and MCS significantly improved in the majority of cats. Almost half of the cats had normal muscle mass already 1 month after 131I treatment. This is awaited evolution after resolution of hyperthyroidism. Serum creatinine was significantly higher in cats with none to mild muscle mass loss compared to cats with moderate to severe muscle mass loss before treatment but this difference was insignificant after treatment (Table 3). These results are in agreement with the well‐known fact that sCr is influenced by muscle mass and sCr might be lower in cats with muscle wasting such as cats with hyperthyroidism or CKD as well as elderly cats.7 There was no significant difference in SDMA in cats with none to mild muscle mass loss when compared to cats with moderate to severe muscle mass loss. This finding is not surprising as in healthy elderly cats and in healthy Beagles, SDMA is not influenced by lean body mass.12, 14
Table 3.
Median (range) serum creatinine and SDMA in cats with none to mild muscle atrophy compared to cats with moderate to severe muscle atrophy
| Variables (units) | None to mild muscle atrophy | Moderate to severe muscle atrophy | Difference between groups: P‐value |
|---|---|---|---|
| Serum creatinine at T0 (mg/dL) | 0.81 (0.54‐1.55) | 0.64 (0.38‐1.04) | .01 |
| Serum SDMA at T0 (μg/dL) | 10.78 (4.00‐18.00) | 9.75 (1.00‐18.00) | .3 |
| Serum creatinine at T1 (mg/dL) | 1.26 (0.69‐2.01) | 1.02 (0.38‐1.70) | .14 |
| Serum SDMA at T1 (μg/dL) | 10.87 (6.00‐19.00) | 11.33 (6.00‐20.00) | .74 |
Abbreviations: SDMA, symmetric dimethylarginine; T0, time before radioiodine treatment; T1, time 1 month after radioiodine treatment.
The majority of cats in our study were euthyroid 1 month after 131I treatment, only 9% cats were subclinically hypothyroid or hypothyroid. None of these hypothyroid cats needed to start thyroid hormone supplementation 1 month after 131I treatment because neither clinical signs of hypothyroidism nor azotemia were present. Percentages of hypothyroidism and transient hypothyroidism after 131I treatment vary in the literature from 5% incidence of iatrogenic hypothyroidism in older studies to 30%, 79%, and 83% in later studies.5, 27, 31, 32 There is a lower incidence of hypothyroidism with low‐dose (2 mCi) compared to standard‐dose (4 mCi) 131I treatment with no significant difference of persistent hyperthyroidism between both groups.21 Despite using mean 131I dose of 142.6 MBq (3.85 mCi), the incidence of hypothyroidism was low (9%) in the present study. This might be because of the short‐term follow‐up of our population, considering that iatrogenic hypothyroidism might develop up to 6‐12 months after 131I treatment. On the other hand, it is also possible that the 4 hypothyroid cats in our study were suffering from transient hypothyroidism which might have resolved in the subsequent months. Furthermore, different cutoff values of TSH to diagnose hypothyroidism varying from 0.15 to 0.30 ng/mL were used in previous studies.5, 21 The RI for euthyroid cats was recently established for the TSH assay specifically used in our study (Immulite Canine TSH).21 We used the upper limit of this RI (0.3 ng/mL) as a cutoff value to determine hypothyroidism. Using this cut‐off value led to diagnosis of a lower number of hypothyroid cats than if we would have used previously reported lower cutoff values. Further studies are needed to establish appropriate cutoff value for diagnosing hypothyroidism in cats.
Previous studies used liquid chromatography‐mass spectroscopy (LC‐MS) which is considered a gold standard technique to measure sSDMA concentrations. In our study, the sSDMA concentrations were measured using a validated immunoassay IDEXX SDMA Test (IDEXX Laboratories, Inc).19 This assay is commercially available and affordable compared to the LC‐MS and consequently more convenient for use in clinics. GFR was performed using plasma exogenous creatinine clearance test as previously described by our research group.9, 20 Glomerular filtration rate RI has to be established for each clearance protocol separately, as there is a high variation depending on the method of GFR measuring, clearance markers, and study population. We used cutoff values for borderline low and low GFR defined by our research group as 1.9 mL/min/kg and 1.4 mL/min/kg, respectively.9
The main limitations of our study were the relatively low number of cats and the absence of azotemia after 131I treatment both of which precluded assessment of predictive value for development of renal azotemia after treatment.
In conclusion, careful interpretation of mildly increased sSDMA with normal sCr in hyperthyroid cats is warranted considering that sSDMA concentrations might return back to RI after resolution of hyperthyroidism in some cats. Additionally, our study shows lower correlation between sSDMA and GFR compared to correlation between sCr and GFR in hyperthyroid cats treated with 131I.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approval was granted by the Ethical Committee of the Faculty of Veterinary Medicine and Bioscience Engineering of Ghent University IACUC, EC 2015/67.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENTS
Study was conducted at the Faculty of Veterinary Medicine, Ghent University, Belgium and supported by IDEXX Laboratories. Results were partially presented at ECVIM‐CA Congress, Malta, 2017 and ECVIM‐CA Congress, Rotterdam, 2018.
Buresova E, Stock E, Paepe D, et al. Assessment of symmetric dimethylarginine as a biomarker of renal function in hyperthyroid cats treated with radioiodine. J Vet Intern Med. 2019;33:516–522. 10.1111/jvim.15407
Funding information IDEXX Laboratories
REFERENCES
- 1. van Hoek I, Daminet S. Interactions between thyroid and kidney function in pathological conditions of these organ systems: a review. Gen Comp Endocrinol. 2009;160:205‐215. [DOI] [PubMed] [Google Scholar]
- 2. Syme HM. Feline hyperthyroidism and renal function In: Bonagura JD, Twedt DC, eds. Kirk's Current Veterinary Therapy XV. St. Louis, MO: Elsevier Saunders; 2014:185‐189. [Google Scholar]
- 3. Williams TL, Peak KJ, Brodbelt D, Elliott J, Syme HM. Survival and the development of azotemia after treatment of hyperthyroid cats. J Vet Intern Med. 2010;24:863‐869. [DOI] [PubMed] [Google Scholar]
- 4. Williams TL, Elliott J, Syme HM. Effect on renal function of restoration of euthyroidism in hyperthyroid cats with iatrogenic hypothyroidism. J Vet Intern Med. 2014;28:1251‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Williams TL, Elliott J, Syme HM. Association of iatrogenic hypothyroidism with azotemia and reduced survival time in cats treated for hyperthyroidism. J Vet Intern Med. 2010;24:1086‐1092. [DOI] [PubMed] [Google Scholar]
- 6. Paepe D, Daminet S. Feline CKD—diagnosis, staging and screening—what is recommended? J Feline Med Surg. 2013;15:15‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hokamp JA, Nabity MB. Renal biomarkers in domestic species. Vet Clin Pathol. 2016;45(1):28‐56. [DOI] [PubMed] [Google Scholar]
- 8. Finch N. Measurement of glomerular filtration rate in cats: methods and advantages over routine markers of renal function. J Feline Med Surg. 2014;16:736‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paepe D, Lefebvre HP, Concordet D, van Hoek I, Croubels S, Daminet S. Simplified methods for estimating glomerular filtration rate in cats and for detection of cats with low or borderline glomerular filtration rate. J Feline Med Surg. 2015;17:889‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kielstein JT, Salpeter SR, Bode‐Boeger SM, Cooke JP, Fliser D. Symmetric dimethylarginine (SDMA) as endogenous marker of renal function. A meta‐analysis. Nephrol Dial Transplant. 2006;21(9):2446‐2451. [DOI] [PubMed] [Google Scholar]
- 11. Braff J, Obare E, Yerramilli M, Elliott J, Yerramilli M. Relationship between serum symmetric dimethylarginine concentration and glomerular filtration rate in cats. J Vet Intern Med. 2014;28(6):1699‐1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hall JA, Yerramilli M, Obare E, Yerramilli M, Yu S, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in healthy geriatric cats fed reduced protein foods enriched with fish oil, L‐carnitine, and medium‐chain triglycerides. Vet J. 2014;202(3):588‐596. [DOI] [PubMed] [Google Scholar]
- 13. Hall JA, Yerramilli M, Obare E, Yerramilli M, Jewell DE. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J Vet Intern Med. 2014;28:1676‐1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall JA, Yerramilli M, Obare E, Yerramilli M, Melendez LD, Jewell DE. Relationship between lean body mass and serum renal biomarkers in healthy dogs. J Vet Intern Med. 2015;29(3):808‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peterson ME, Varela FV, Rishniw M, Polzin DJ. Evaluation of serum symmetric dimethylarginine concentration as a marker for masked chronic kidney disease in cats with hyperthyroidism. J Vet Intern Med. 2018;32(1):295‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Peterson ME, Becker DV. Radioiodine treatment of 524 cats with hyperthyroidism. J Am Vet Med Assoc. 1995;207:1422‐1428. [PubMed] [Google Scholar]
- 17. Paepe D, Verjans G, Duchateau L, Piron K, Ghys L, Daminet S. Routine health screening: findings in apparently healthy middle‐aged and old cats. J Feline Med Surg. 2013;15:8‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med. 2007;21:542‐558. [DOI] [PubMed] [Google Scholar]
- 19. Prusevich P, Patch D, Obare E, et al. Validation of a novel high throughput immunoassay for the quantitation of symmetric dimethylarginine (SDMA) [American Association for Clinical Chemistry 2015, abstract B‐048]. Clin Chem. 2015;16:S135. [Google Scholar]
- 20. Van Hoek I, Vandermeulen E, Duchateau L, et al. Comparison and reproducibility of plasma clearance of exogenous creatinine, exo‐iohexol, endo‐iohexol, and 51Cr‐EDTA in young adult and aged healthy cats. J Vet Intern Med. 2007;21:950‐958. [DOI] [PubMed] [Google Scholar]
- 21. Lucy JM, Peterson ME, Randolph JF, et al. Efficacy of low‐dose (2 millicurie) versus standard‐dose (4 millicurie) radioiodine treatment for cats with mild‐to‐moderate hyperthyroidism. J Vet Intern Med. 2017;31(2):326‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vaske HH, Armbrust L, Zicker SC, et al. Hyperthyroid cats managed with a controlled iodine diet. Int J Appl Res Vet Med. 2016;14(1):38‐48. [Google Scholar]
- 23. Corsini A, Crosara S, Carotenuto G, et al. Symmetric dimethylarginine (SDMA) in hyperthyroid cats. Research Communications of the 27 ECVIM‐CA, ESVE‐P‐3 . J Vet Intern Med. 2018;32:525‐609. [Google Scholar]
- 24. Ittermann T, Bahls M, Atzler D, et al. L‐arginine derivatives are associated with the hyperthyroid state in the general population. Thyroid. 2016;26(2):212‐218. [DOI] [PubMed] [Google Scholar]
- 25. Siren MP, van‐der‐Sijp JR, Teerlink T, et al. The human liver clears both asymmetric and symmetric dimethylarginine. Hepatology. 2005;41:559‐565. [DOI] [PubMed] [Google Scholar]
- 26. van Hoek I, Lefebvre HP, Peremans K, et al. Short‐ and long‐term follow‐up of glomerular and tubular renal markers of kidney function in hyperthyroid cats after treatment with radioiodine. Domest Anim Endocrinol. 2009;36(1):45‐56. [DOI] [PubMed] [Google Scholar]
- 27. Boag AK, Neiger R, Slater L, Stevens KB, Haller M, Church DB. Changes in the glomerular filtration rate of 27 cats with hyperthyroidism after treatment with radioactive iodine. Vet Rec. 2007;161:711‐715. [DOI] [PubMed] [Google Scholar]
- 28. Jepson R, Brodbelt D, Vallance C, et al. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med. 2009;23:806‐813. [DOI] [PubMed] [Google Scholar]
- 29. Ross LA, Finco DR. Relationship of selected clinical renal function tests to glomerular filtration rate and renal blood flow in cats. Am J Vet Res. 1981;42:1704‐1710. [PubMed] [Google Scholar]
- 30. Haller M, Rohner K, Muller W, et al. Single‐injection inulin clearance for routine measurement of glomerular filtration rate in cats. J Feline Med Surg. 2003;5:175‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peterson ME. Radioiodine treatment of hyperthyroidism. Clin Tech Small Anim Pract. 2006;21(1):34‐39. [DOI] [PubMed] [Google Scholar]
- 32. Nykamp SG, Dykes NL, Zarfoss MK, Scarlett JM. Association of the risk of development of hypothyroidism after iodine 131 treatment with the pretreatment pattern of sodium pertechnetate Tc 99m uptake in the thyroid gland in cats with hyperthyroidism: 165 cases (1990‐2002). J Am Vet Med Assoc. 2005;226:1671‐1675. [DOI] [PubMed] [Google Scholar]


