Figure 2. NOS2 promoter transactivation and mRNA is suppressed by expression of dnHMGA1.
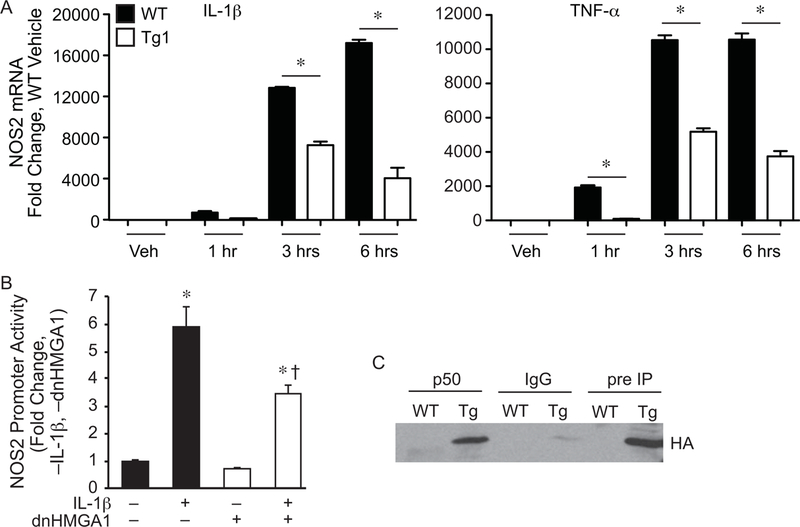
A) SMCs harvested from WT or Tg1 mice were exposed to vehicle (Veh), IL-1β (10 ng/ml, left panel), or TNF-α (10 ng/ml, right panel) for various time points (as depicted). Total RNA was extracted from the cells, and levels of NOS2 were measured by qRT-PCR. Data are presented as mean ± SEM, n=3 per group, with testing by one-way ANOVA (P<0.0001). Significant comparisons; * WT versus Tg1. B) SMCs were transiently transfected with a NOS2 luciferase reporter plasmid (base pairs −1485/+31), and either a vector control plasmid or an expression plasmid for dnHMGA1. An expression plasmid for β-galactosidase was used to correct for transfection efficiency. Cells were allowed to recover overnight and then the cells were exposed to IL-1β (10 ng/ml) or vehicle for 24 hours prior to harvest. Luciferase activity of each group is presented as mean ± SEM, n=6 per group, with testing by one-way ANOVA (P<0.0001). Significant comparisons; * versus no (–) IL-1β and no (–) dnHMGA1, and † versus IL-1β (+) and no (–) dnHMGA1. C) Co-immunoprecipitation assay (co-IP) was performed as described in the Material and Methods. SMCs from WT or dnHMGA1 Tg mice were then harvested and the nuclear protein extracts were incubated with p50 antibody-crosslinked protein A/G beads, rabbit IgG-crosslinked protein A/G beads, or protein A/G beads alone. The bound proteins were separated with SDS-PAGE. Western blot analysis of IP bound proteins (lanes 1 to 4) and pre-IP cellular proteins (lanes 5 and 6) were performed using HA antibody.
