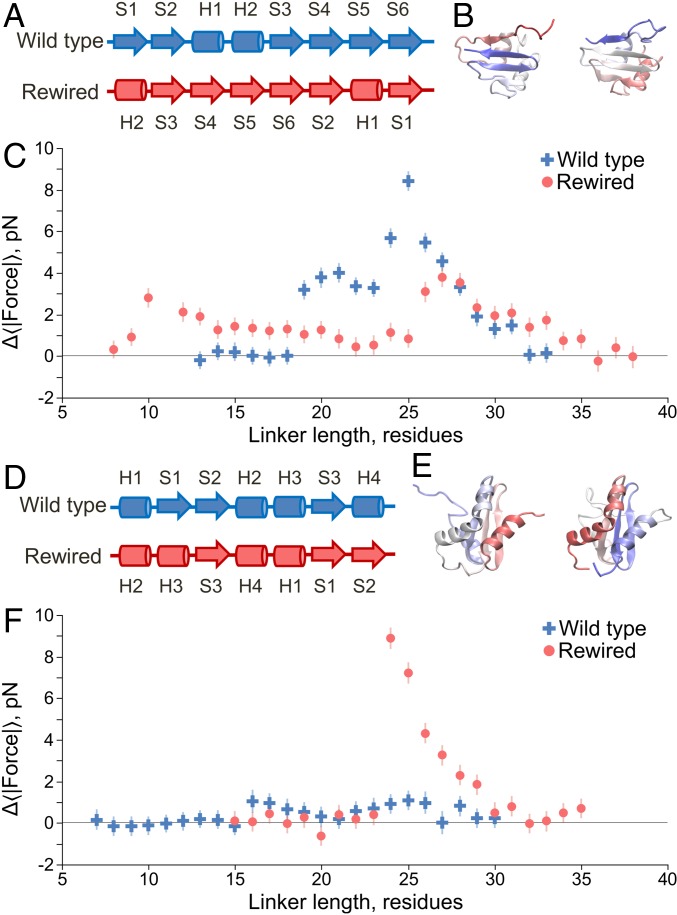
Fig. 5.
Topology influences the pulling force, with C-terminal β hairpins giving rise to large forces. (A) Linear secondary structure representation, from N to C terminus, of the domain 1P9K. Arrows indicate β strands, and cylinders indicate α helices. The original wild-type structure is shown at the top, whereas the rewired mutant is shown below. The rewired mutant maintains the same secondary structure labels (H1, S1, etc.) as the wild type to illustrate how their relative positioning has changed along the primary structure due to rewiring. (B) Crystal structures of the wild-type (Left) and rewired (Right) domains, with the N terminii in red and the C terminii in blue. (C) Pulling force (Eq. 6) versus linker length for the wild-type (blue crosses) and rewired mutant (red circles) domains at 310 K. Moving the C-terminal β hairpin (formed by strands S5–S6) to the middle of the domain generates a lower maximum force in the rewired mutant. (D) Same as A except for protein 1NY8, which in the wild type has no C-terminal β hairpin but in the rewired mutant has one formed by strands S1–S2. (E) Same as B except for protein 1NY8. (F) Same as C but for protein 1NY8; wild-type results are blue crosses, and mutant results are red circles. Error bars represent 95% confidence intervals about the mean calculated from block averaging.