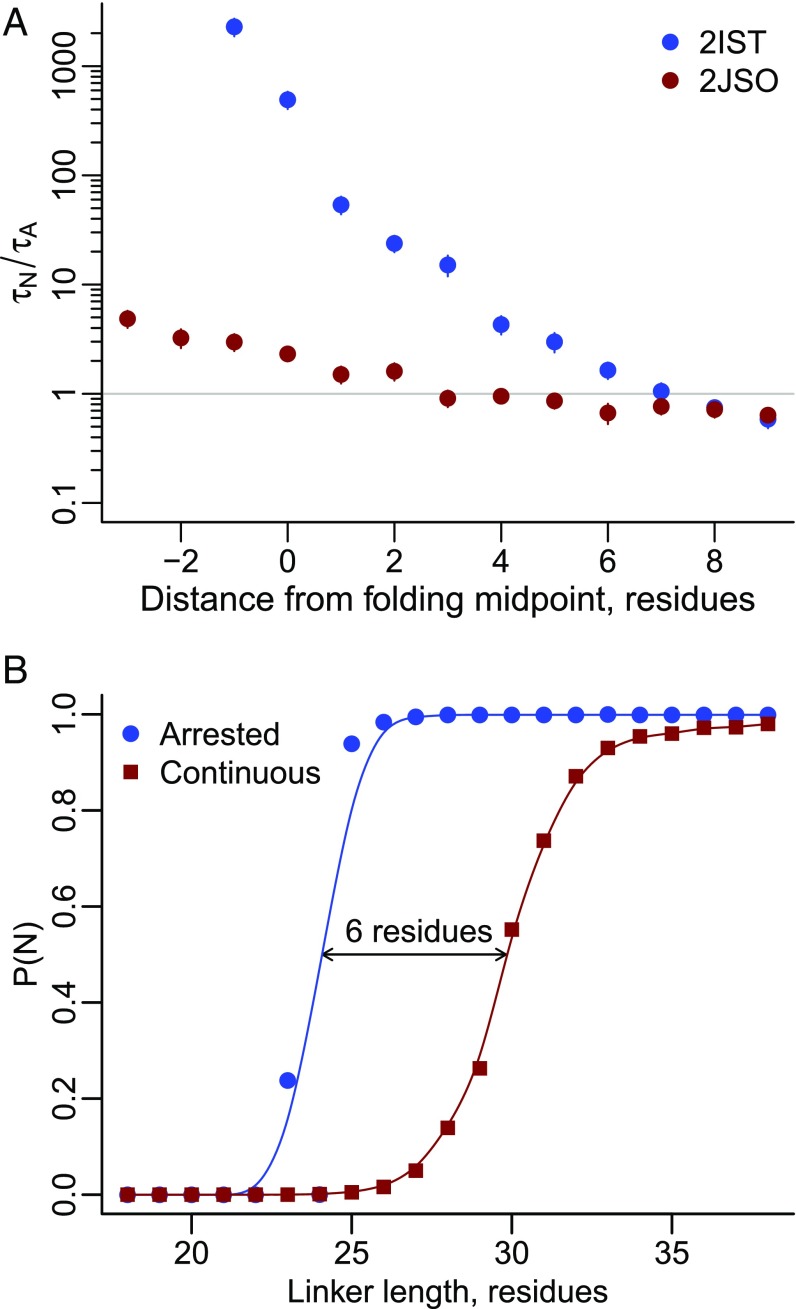
Fig. 8.
A delay in cotranslational folding due to continuous synthesis causes 2IST’s pulling force to vanish. (A) The ratio of folding time to amino acid addition time versus linker length for proteins 2IST (blue circles) and 2JSO (red circles) as calculated from temperature quenching simulations on arrested ribosomes. Although 2IST folds faster in bulk solution, its folding rate slows at short linker lengths on the ribosome. Error bars represent 95% confidence intervals about the mean, calculated from bootstrapping. (B) Probability of folding versus linker length for protein 2IST on arrested ribosomes (blue circles) and undergoing continuous synthesis (red squares). The longer folding time for 2IST causes the protein to fold six residues later during continuous synthesis than on an arrested ribosome.