Abstract
With progress in sensors and communication technologies, the range of sleep monitoring is extending from professional clinics into our usual home environments. Information from conventional overnight polysomnographic recordings can be derived from much simpler devices and methods. The gold standard of sleep monitoring is laboratory polysomnography, which classifies brain states based mainly on EEGs. Single-channel EEGs have been used for sleep stage scoring with accuracies of 84.9%. Actigraphy can estimate sleep efficiency with an accuracy of 86.0%. Sleep scoring based on respiratory dynamics provides accuracies of 89.2% and 70.9% for identifying sleep stages and sleep efficiency, respectively, and a correlation coefficient of 0.94 for apnea–hypopnea detection. Modulation of autonomic balance during the sleep stages are well recognized and widely used for simpler sleep scoring and sleep parameter estimation. This modulation can be recorded by several types of cardiovascular measurements, including ECG, PPG, BCG, and PAT, and the results showed accuracies up to 96.5% and 92.5% for sleep efficiency and OSA severity detection, respectively. Instead of using recordings for the entire night, less than 5 min ECG recordings have used for sleep efficiency and AHI estimation and resulted in high correlations of 0.94 and 0.99, respectively. These methods are based on their own models that relate sleep dynamics with a limited number of biological signals. Parameters representing sleep quality and disturbed breathing are estimated with high accuracies that are close to the results obtained by polysomnography. These unconstrained technologies, making sleep monitoring easier and simpler, will enhance qualities of life by expanding the range of ubiquitous healthcare.
Keywords: Sleep monitoring, Unconstrained, Nonintrusive, Polysomnography, Ubiquitous healthcare
Introduction
We spend almost one third of our lifetime asleep, and sleep is indispensable to life, like air, water, and food. Disturbed or reduced quality of sleep results in daytime solemnness, which increases the risk of accidents and deteriorates the mental and physical qualities of daytime activities. Insomnia, a condition in which a person is unable to fall asleep, and sleep breathing disorders, which interrupt ordinary breathing repetitively during sleep, are the most common sleep disorders. Narcolepsy, periodic limb movement syndrome, REM sleep disorder, and parasomnias are sleep-related disorders that are also frequently encountered. In addition, it is widely reported that sleep disturbances increase the comorbidity of chronic diseases in healthy subjects. Shorter or longer sleep durations have been reported to increase the likelihood of developing diabetes by two to three times [1]. Prolonged short sleep durations in healthy subjects can elevate blood pressure equilibrium and increase the hazard ratio of hypertension more than two times [2]. Sleep deficits predicted depression symptoms across a 6‐month follow‐up [3]. Meta-analysis revealed a consistent increased risk of obesity amongst short sleepers in children as well as in adult populations [4].
Full-night sleep monitoring and accurate evaluation is critical for the diagnosis and therapy of sleep-related disorders and for the analysis and prediction of mental and behavioral performances. Polysomnography (PSG) [5], which comprehensively records physiological changes that occur during sleep, and scoring based on the American Academy of Sleep Medicine (AASM) scoring manual [6] are the gold standard methods in sleep medicine for sleep monitoring. However, PSG is performed in sleep clinics with specialized equipment and trained personnel. This makes PSG expensive and limits its accessibility. For sleep evaluation at home in non-specific subjects, PSG is impractical. Hence, studies to find alternative methods to measure sleep objectively in non-laboratory settings comparable to PSG have been widely attempted [7]. This paper reviews and summarizes the endeavors toward convenient monitoring of sleep in home environments.
Sleep monitoring methods
While PSG is the gold standard for sleep monitoring and evaluation of related behaviors, many studies have sought to monitor sleep in simpler ways. These methods are also based on biological signals, which vary accordingly during the sleep stages and associated events occurring during sleep. In this paper we classified the sleep monitoring methods based on the major biological signals that the methods are based upon and reviewed the studies performed compared to standard PSG.
EEG based sleep monitoring
Sleep affects almost every part of body, but the brain performs the central role in controlling all the dynamics accompanying sleep, including consciousness. Thus, sleep monitoring begins by monitoring changes in brain activity using an electroencephalogram (EEG) during sleep periods. Practically, EEGs are primarily used to classifying sleep stages in regular PSG studies for sleep medicine.
Laboratory PSG
Polysomnography in sleep laboratory is typically done with a minimum of 12 channels of biological signals. At least three channels of EEGs, two channels of electrooculogram (EOG), and one channel of chin electromyogram (EMG) are compulsory. The AASM recommended channels also include for airflow measured with nasal pressure or thermal device, respiratory efforts of chest and upper abdominal wall measured with respiratory inductance plethysmography (RIP) or piezoelectric belt, leg movement measured by EMG, oxygen saturation, heart rhythms measured by ECG, and body position [6]. The number of channels and combination of recording signals vary in every laboratory and their targeted patients. Sleep stages are defined based on EEG signals in conjunction with EOG and chin EMG. Other channels are used mainly for detecting events like sleep apnea and leg movement. To record the PSG, about twenty electrodes and sensors are attached to the subject and an equal number of wires are connected to the recording unit. Thus, PSG is performed with professional staff attended. Table 1 summarizes the major sleep parameters usually derived from laboratory PSG for clinical use.
Table 1.
Representative sleep parameters derived by laboratory polysomnography
| Type | Parameter | Abbreviation | Derivation |
|---|---|---|---|
| Sleep scoring data | Total recording time | TRT | Time from “light out” to “light on”, in min |
| Total sleep time | TST | Time spent in N1 + N2 + N3 + R, in min | |
| Sleep latency | SL | Time from “light out” to 1st epoch of any sleep, in min | |
| REM sleep latency | RL | Time from sleep onset to 1st epoch of stage R, in min | |
| Wake after sleep onset | WASO | TRT-SL-TST, in min | |
| Sleep efficiency | SE | (TST/TRT) × 100 | |
| Time in each stage | N1, N2, N3, R | Time in each of N1, N2, N3, and R stages, in min | |
| Percent in each stage | N1%, N2%, N3%, R% | (Time in each stage/TST) × 100 | |
| Movement events | Number of periodic limb movements of sleep | PLMS | Number of limb movements during TST |
| PLMS index | PLMSI | (PLMS/TST) × 60, number of movements per hour | |
| Respiratory events | Number of obstructive sleep apneas | #OSA | Number of obstructive apneas during TST |
| Number of central sleep apneas | #CSA | Number of central apneas during TST | |
| Number of mixed sleep apneas | #MSA | Number of mixed apneas during TST | |
| Number of hypopneas | #SH | Number of hypopneas during TST | |
| Number of sleep apneas | #SA | #OSA + #CSA + #MSA | |
| Apnea index | AI | (#SA/TST) × 60, number of apneas per hour | |
| Hypopnea index | HI | (#SH/TST) × 60, number of hypopneas per hour | |
| Apnea–hypopnea index | AHI | ((#SA + #SH)/TST) × 60, number of apneas and hypopneas per hour | |
| Number of oxygen desaturations | #OD | Number of oxygen desaturations ≥ 3% or 4% during TST | |
| Oxygen desaturation index | ODI | (#OD/TST) × 60, number of oxygen desaturations per hour |
Sleep stages are divided into five classes: W, N1, N2, N3, and R sleep. Stage W is the state of wakefulness before and during the sleep. Stage N1 is the lightest sleep stage of non-REM (NREM) sleep defined by low amplitude mixed frequency EEG activity and usually accounts for less than 10% of the total sleep period. Stage N2 is the light sleep state defined by the recording of EEG with K-complex and sleep spindle. Stage N2 takes the largest portion of sleep, usually accounting for about 50% of the total sleep period. Stage N1 and N2 are also collectively called “light” sleep. Stage N3 is the deepest state of sleep defined by high amplitude slow delta waves in the EEG and accounts for less than 20% of the total sleep time. N3 is also called “deep” sleep or slow wave sleep (SWS). Stages N1, N2, and N3 are collectively called NREM sleep. Stage R is the sleep state defined by rapid eye movement (REM), recorded in an EOG, and accounts for about 20% of the total sleep time. Stage R is the period during which dreaming occurs and is also called REM sleep. The sleep scoring parameters include total recording time (TRT), total sleep time (TST), sleep latency (SL), REM sleep latency (RL), wake after sleep onset (WASO), sleep efficiency (SE), and time and percentage spent in each stage of sleep, as summarized in Table 1.
In addition to sleep scoring data, parameters for associated events are also derived from PSG. The number and index of periodic limb movements are parameters representing movement events during sleep. Apnea and hypopnea are the major respiratory events occurring during sleep. Apnea is the temporary cessation of breathing and hypopnea is overly shallow breathing. Recording segments are scored as apnea when there is a drop of peak signal excursion ≥ 90% compared to the pre-event baseline in airflow signal and it lasts more than 10 s. Apnea is classified into three classes depending on the existence of co-occurring inspiratory effort. If there is a co-occurring inspiratory effort, it is scored as obstructive sleep apnea (OSA). If inspiratory effort is absent, it is scored as central sleep apnea (CSA) and if inspiratory effort is present partially, it is scored as mixed sleep apnea (MSA). Hypopnea is scored when peak excursion drop ≥ 30% last more than 10 s and there is ≥ 3% (or 4%) oxygen desaturation from pre-event baseline. Respiratory event parameters, including the apnea–hypopnea index (AHI), reflect the severity of breathing abnormalities by the rates and types of apnea, hypopnea, and oxygen desaturation events.
While PSG is the gold standard for sleep monitoring in medical practice, its complexity and inconvenience greatly limits its application and prevents the expansion of sleep studies into a wider range outside of hospital environments. Thus, methods to simplify sleep monitoring while generating comparable information have been widely studied. Since the EEG is the most difficult to obtain in practice, many studies have been performed to evaluate sleep without using EEG channels and with greatly reduced numbers of channels.
Ambulatory PSG
An ambulatory or portable PSG is a device that can perform a full PSG outside of the laboratory. It has at least seven channels, including EEG [8]. The major difference from devices used for laboratory PSG, is that overnight recording is performed without the presence of a technologist. These devices are called comprehensive portable devices. A study compared the validity of a portable PSG against a laboratory PSG from the same manufacturer [9]. There was good agreement between portable PSG and laboratory PSG for sleep scoring parameters and the apnea–hypopnea index (r = 0.99), and 90% of the portable recordings were satisfactory for clinical interpretation. This unattended PSG has also used to compare sleep at home with sleep in a laboratory in a population suspected to have OSA [10]. While the results showed equivalent statistics for AHI, sleep monitoring at home showed better sleep quality with increased SE (82% vs. 75%), longer TST (412 vs. 365 min), shorter SOL (28 vs. 45 min), and more time in REM sleep (19% vs. 16%) over sleep monitoring in a laboratory. These results imply the sleep in a laboratory is not same as sleep at home, and sleep monitoring at home is preferable because the data is closer to a subject’s natural and ordinary sleep behavior. Portable PSG is considered to be a viable alternative to laboratory PSG, but it requires the involvement of professional staff for sensor attachment, limiting the use of the device for wider home application.
Sleep monitoring with EEG only
Since the EEG is the main tool used in sleep stage scoring, we might question how accurately we can evaluate sleep without using other types of signals. Without using EMG and EOG, a single channel EEG of Cz-Pz measured during PSG was used for automatic scoring of sleep stages. Compared to the laboratory PSG, overall epoch-by-epoch agreements were 96.0% (2 classes: Wake/Sleep), 92.1% (3 classes: Wake/REM/NREM), 84.9% (4 classes: Wake/REM/Light/Deep), and 82.9% (5 classes: wake/REM/N1/N2/N3) [11]. By combining multiscale entropy (MSE) and autoregressive (AR) models [12], the epoch-by-epoch overall agreement for the 5-stage classification improved up to 85.4% compared to PSG. A headband containing fabric sensors to measure a single channel EEG around the Fp1-Fp2 location was used for sleep stage estimation [13]. The measured signal includes contributions from eye movements and the frontalis muscle. The device scored four stages of sleep with a 75.3% agreement rate compared to the PSG score and discriminated sleep from wake states with 91.9% accuracy, which is better than the 86.0% accuracy of the actigraphy device used simultaneously. As a minimally obtrusive method for recording EEGs, ear-EEG devices using electrodes in the ear have been proposed and applied for sleep stage scoring [14]. Using the same algorithm applied to single channel EEG, they showed 82.0% average agreement for 5-stage classification, comparable to the 85.7% agreement of a single surface EEG.
Movement-based sleep monitoring
Physical quiescence is the first recognizable dominant feature of sleep before any other accompanying physiological modulations occur. Thus, an effort to evaluate the sleep as an alternative or complementary method to PSG has begun with monitoring of physical inactivity during sleep. With miniaturized, low-cost acceleration and gyro sensors, monitoring sleep by movement has become the easiest method, despite its inherent limitations.
Actigraphy
Actigraphy (ACT) is a method for collecting data generated by body movement using a wrist-watch type device. Most of recent devices use a solid-state tri-axis linear accelerometer as a measurement sensor. They have their own algorithms to evaluate activity levels, movement patterns, and sleep measures within predefined time windows. Since the American Sleep Disorders Association (ASDA) guidelines [15] were created in 1995, actigraphy has been accepted as a reliable and valid sleep assessment method by sleep researchers and sleep medicine clinicians [16] to assist in the evaluation of patients with circadian disorders, sleep–wake disturbances, and insomnia [17]. Use of ACT devices has continuously increased in practice and research, and they have become common devices for sleep–wake evaluation [18]. Generally, ACT is reported with high sensitivities of over 90% for recognizing sleep, but rather low specificities of less than 60% for detecting wake-states. In a study [19] of 77 subjects including young and older adults, healthy or chronic primary insomniac (PI) patients, and night-workers that sleep during the day, sensitivity (0.97) and accuracy (0.86) were high, whereas specificity (0.33) was low. Performance was only slightly modified by gender, insomnia, and day/night sleep timing. Increased age slightly reduced specificity. This low specificity is caused by quiet wakefulness or periods of lying down, which are recognized as sleep periods due to the absence of the movement. Thus, ACT has a tendency to overestimate sleep time and underestimate wake time and sleep latency. These characteristics produce increased discrepancies when they are applied to groups having increased wake time or sleep fragmentation compared to the normal population.
ACT has been shown to be the most effective method for deriving the effects of various behavioral and medical interventions on sleep–wake patterns [20]. Since it is a wrist-worn device, it can be used throughout an entire day, including bedtime, and for several days. Thus, ACT is suitable for the assessment of circadian rhythm-related disorders and longitudinal sleep–wake patterns. In summary, ACT measures physical variations rather than physiological variations caused by sleep and can only discriminate between sleep and wake stages but not the various sleep stages.
Mobile and wearable devices
Wide acceptance of ACT as a method for evaluating sleep–wake patterns in sleep studies has allowed many types of electronic devices to be used as a sleep monitoring tools. Six-axis solid-state miniaturized sensors, tri-axis linear accelerometers, and tri-axis gyroscopes are included as basic sensors in most recent mobile or wearable devices, like health trackers, smart watches, and smart phones. Thus, sleep monitoring has been integrated into the device functions with their own evaluation software. Some papers [21–23] reviewed the application of these devices for sleep monitoring.
Mobile devices, like smartphone or tablets, use their built-in sensors to monitor sleep by positioning the device simply on bed surface. When placed on the bed surface, the built-in accelerometer detects lack of movement as an indicator of sleep. Hundreds of sleep-related apps are available through the Apple or Android app stores [23]. Many questions surround the clinical significance of these apps, and their applications have limited to health-related domains, like facilitation of sleep, pleasant sleep wakening, self-guided sleep assessment, and social connections.
In contrast to mobile devices, wearable units are applied directly to the body on the wrist, neck, or clothing. Many types of health trackers and smart watches belong to this category. Even though a sleeper must wear the unit, which may cause discomfort, it tracks body movements with presumably increased accuracy via solid attachment to the wearer. They can provide ACT equivalent accuracy in sleep–wake discrimination. Mantua et al. [24] compared the reliability of sleep measures from four wearable devices, Basis Health Tracker, Misfit Shine, Fitbit Flex, and Withings Pulse O2, to the research-based ACT device, Actiwatch Spectrum, and to PSG. They found no differences between these devices and a strong correlation with PSG, higher than 0.84, for total sleep time (TST) estimation. However, for sleep efficiency (SE), only mean values from the Actiwatch correlated with PSG, yet this correlation was weak. Studies that validated ACT confirmed that overestimating sleep by detecting quiet wakefulness as sleep is a general drawback of movement-based sleep measurements.
Environmental devices
Movement during sleep can be recognized even without attaching sensors directly to the body by installing sensors in the user’s native sleeping environment. These types of devices have the advantages of being unobtrusive and adequate for longer term monitoring without the user’s intervention.
Bed actigraphy (BACT) was implemented by installing four load-sensing cells supporting the bed [25] for sleep–wake discrimination. When BACT was compared with ACT and PSG, mean epoch-by-epoch agreements between the BACT and PSG and BACT and ACT recordings were 95.2 and 94.3% respectively. The mean absolute differences in sleep efficiency (SE) estimation were 1.8 ± 0.82 and 1.9 ± 1.16% respectively. BACT showed better performance than ACT in differentiating “wake” from “sleep” stages and proved to be sufficiently robust and comparable to PSG analysis.
Polyvinylidene fluoride (PVDF) films are a very thin and flexible films that are widely used as film transducers or speaker elements. These films are also appropriate for unobtrusively detecting mechanical loads caused by movements in bed. Thin PVDF film sensors located just under the bed cover sheet were used for sleep detection [26]. Based on body movement recordings, these sensors estimated sleep with 92.9% sensitivity, 62.0% specificity, and 89.2% accuracy for subjects whose sleep efficiency is higher than 80%.
Modulated Doppler radar of 5.8 GHz was employed [27] for movement detection and identified sleep with 88%, 69% and 82% in sensitivity, specificity, and accuracy, respectively, compared with PSG. The increased specificity compared to that of ACT is attributed to the radar reflecting from larger body parts instead of relying on the motion of a single wrist as in ACT.
Non-contact monitoring using near-infrared video was used to measure the quality of sleep [28]. Activity levels were identified by the accumulated rectified frame differences from low resolution videos (160 × 120) and of low signal to noise ratio (SNR) obtained in darkened room. The accuracy of estimating wake/sleep status relative to PSG was 92.1%, while the wrist ACT was 91.2%. Video methods have also been used for diagnosing attention-deficit hyperactivity disorder (ADHD) in children during sleep [29]. When the duration of gross body movement and resting without movement were compared, children with ADHD showed more frequent movement and shorter resting durations than normally developed children.
Respiration-based sleep monitoring
Respiration is another biological signal widely using in sleep monitoring next to EEGs. The first concern of respiration monitoring is to identify the abnormal respiratory events of apnea/hypopnea and evaluate the severity of these breathing disorders. Obstructive sleep apnea is a common and serious chronic breathing disorder characterized by repeated stops and starts of breathing due to upper airway collapse during sleep. In addition to its acknowledged effect on nocturnal sleep quality and ensuing daytime fatigue and sleepiness, it is also recognized as an independent risk factor for several clinical consequences, including systemic hypertension, cardiovascular disease, stroke, and abnormal glucose metabolism. Its prevalence is estimated to be in the range of 3–7% of the population and increases in certain subgroups of the population bearing higher risk factors [30]. Laboratory PSG includes multiple channels for monitoring airflow and respiratory effort signals and is a standard tool for diagnosing and evaluating the severity of sleep apnea syndrome.
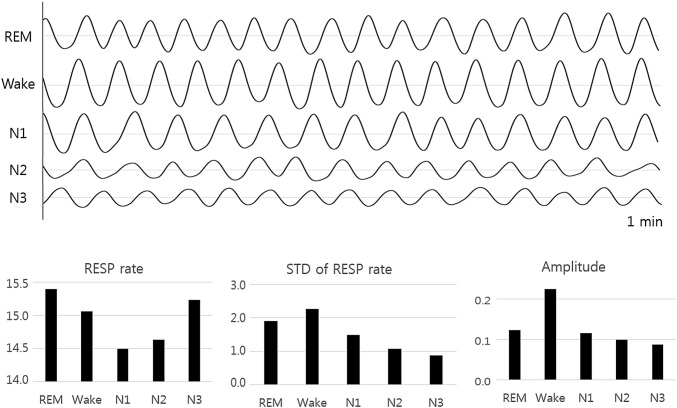
While the breathing is uninterrupted, its amplitude changes as the sleep becomes deeper with the modification of the homeostasis of partial pressures of arterial oxygen (PaO2) and carbon dioxide (PCO2) and pH levels. Without the volitional influences that appear during wakefulness, these changes differ significantly with specific sleep stages [31]. Figure 1 shows the variation of respiratory frequency and amplitude for the five sleep stages. Minute ventilation and tidal volume decrease as sleep progresses from awake to light sleep, slow wave sleep (SWS), and finally, rapid eye movement (REM) sleep [32]. Respiration during NREM sleep demonstrates an inherently more regular respiratory pattern than wakeful breathing with similar mean frequencies. In contrast, respiration during REM sleep is typically characterized by an increased frequency and a reduced regularity [31]. These variations of breathing patterns with sleep stage create the possibility of using respiration signals as an alternative method to evaluate sleep stages in an unobtrusive and simpler way at home environment.
Fig. 1.
Variation of respiration rate and amplitude of sleep stages. The upper part shows the recorded respiratory signals during 1 min for each sleep stage and the lower part shows the calculated average respiration rate, standard deviation of respiration rate, and average amplitude for each sleep stage
Home sleep apnea testing
Home sleep apnea testing (HSAT) is a simplified method for providing the information required to diagnose OSA. AASM published clinical guidelines in 2007 and scoring criteria in 2015. AASM recommends HSAT utilizing respiratory airflow and/or respiratory effort parameters or utilizing peripheral arterial tonometry (PAT). This allows subjects to sleep conveniently at home while wearing equipment that collects information about how they breathe instead of staying in a sleep laboratory overnight for regular PSG. HSAT is supposed to report number of respiratory events and respiratory event index (REI), which is an alternative to AHI, calculated based on HSAT measured during sleep [6]. This method includes channels for blood oxygen levels and heart rate monitoring.
While HSAT provide information on respiratory events, it does not provide the information on each of the sleep stages. Thus, the use of HSAT is targeted only at subjects suspected of having moderate to severe OSA without having another sleep disorder or other critical medical conditions. The simple two-channel HSAT device has a sensitivity of 80% and specificity of 83% for detecting OSA in unattended home settings compared to laboratory PSG results [33]. The overall performance of the HSAT device is considered to be acceptable to confidently “rule-in” OSA in a population with a high probability of OSA. Nonetheless, enhancements in the estimation of total sleep time (TST) without using an EEG is required to estimate AHI more accurately.
Sleep stage estimation with respiratory signals
Based on the increased and irregular respiratory patterns during REM sleep, REM sleep was estimated with an accuracy of 89% and a Cohen’s kappa coefficient (κ) of 0.65 by calculating the average rate and variability of respiration rate during each epoch and comparing those values with their smoothed signals [34]. In this study, they used two channels for respiration signals from thermocouple and belt-type sensors measured during PSG. However, respiration is accompanied by body movements and thus it can be measured by several unobtrusive methods, and sleep stages also can be derived from these respiration signals.
Respiratory movement measured unobtrusively with 5.8 GHz Doppler radar has been used for sleep stage estimation [27]. For periods where there was no significant limb or torso movement, respiratory-related movement was the predominant recorded signal. Sleep stages were estimated based on breathing frequency and relative amplitude using a peak and trough identifying algorithm. Accuracies of 69, 82, 61, 87, 97, and 98% for Wake, REM, N1, N2, N3, and N4 sleep stages were reported, respectively, for 14 normal subjects compared to simultaneously recorded PSG results [27].
Pressure images obtained from pressure-sensitive bed sheets [35] fabricated by sandwiching a thin piezo-resistive fabric between two sheets of e-textile fabric were used to record respiratory movements and estimate sleep stages. When the subject lies on the bed sheet, the inhaling and exhaling movements of the diaphragm causes pressure changes that are recorded. Firstly, NREM sleep was recognized based on the regularity of the amplitude and frequency in the recorded respiration movements. Since respiratory signals showed the same irregularity with clear variations in the amplitude and frequency during REM and Wake stages, REM sleep was recognized after rejecting the Wake stage based on the detected body movement for the remaining epochs. Results showed 72.2% accuracy for three stages (Wake, NREM, and REM) classification compared to PSG measurements.
With PVDF film sensors located just beneath the bed cover sheet, apneic events were detected with an accuracy of 85%, and the estimated AHI showed a correlation of 0.94 with the AHI derived from PSG [36]. A PVDF film sensor size of 30 × 30 cm used for body movement detection was also used for estimating sleep stages unobtrusively based on recorded respiratory movements [26]. The respiratory movements were extracted from the PVDF data, and four stages of sleep (Wake, Light, SWS, and REM) were estimated based on the relative magnitudes and variabilities in the respiratory frequency. REM sleep was recognized by increases in the average respiration frequency and its variability. The epochs were determined to be SWS if the variability of respiratory frequency was lower than a threshold, which was calculated from the smoothed respiratory frequency over 10 epochs. Epochs not scored with REM sleep or SWS were regarded as light sleep. Compared to PSG results, the method classified the four sleep stages with an average accuracy of 70.9% and kappa of 0.48, which are comparable to those of ambulatory PSG devices. No significant differences were observed in the detection performances between the 12 normal and 13 OSA subject groups [26].
Heart rate-based sleep monitoring
Heart rate modulation during sleep
Modulation of autonomic nerve balance during sleep has been studied and confirmed in many studies [37–41]. Blood pressure (BP) and heart rate (HR) declined significantly as the sleep became deeper during NREM sleep from wakefulness to N1, N2, and finally, to the SWS stage, while during REM sleep, they returned to levels similar to wakefulness [38]. Simultaneously recorded sympathetic-nerve activity (SNA), measured using tungsten microelectrodes inserted into the sympathetic-nerve fascicles, also declined like BP and HR during the NREM sleep period, but increased during REM sleep to values 215% higher than the wakefulness level in normal subjects. Sympathetic predominance that characterizes wakefulness decreases during NREM sleep, becomes minimal in SWS and rapidly increases toward mean Wake levels during REM sleep.
Heart rate variability (HRV) is used to investigate the modulation of autonomic nerve activity as an efficient, noninvasive and unobtrusive method. Variations of HRV during sleep stages was well reviewed [42]. Among several heart rate variability (HRV) parameters, a low-frequency (LF) power representing sympathetic and parasympathetic influences and a high-frequency (HF) power of parasympathetic origin are the most representative. The sympathovagal balance can be defined as the ratio LF/HF. The study revealed a decrease in LF during NREM sleep, with minimal values during SWS and elevated levels similar to those of wakefulness during REM sleep [39]. HF increased with sleep onset, reaching maximal values during N3 sleep. The LF/HF ratio changed accordingly, showing a similar pattern to LF and shifted toward parasympathetic predominance during N3 sleep.
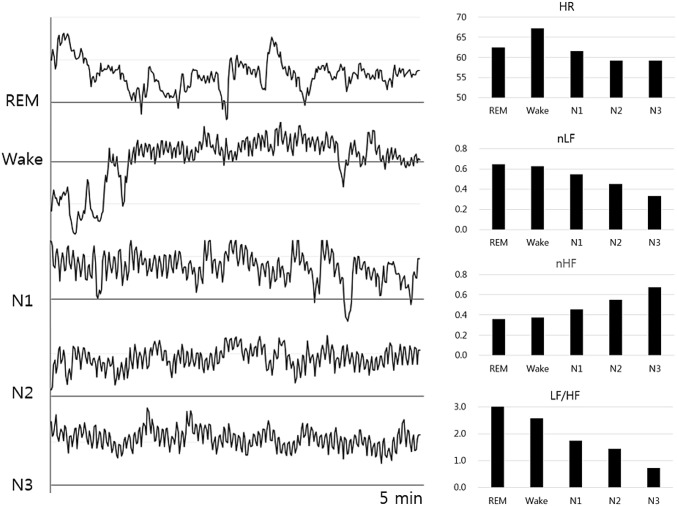
Figure 2 shows the calculated HRV parameters from 72 heathy subject data collected during past polysomnography experiments in the authors’ laboratory. The figures clearly show the overall activation of parasympathetic rhythms and deactivation of sympathetic rhythms when the stages move from REM sleep to deep sleep through the Wake and light sleep stages.
Fig. 2.
Variation of heart rate variability (HRV) parameters during the sleep stages. The left part shows an example of the variation of heart beat intervals over 5 min for each sleep stage. The right part shows the average HRV parameters for each sleep stage. HR heart rate, nLF normalized low-frequency power, nHF normalized high-frequency power, LF/HF ratio of low-frequency power to high-frequency power
These noninvasive HRV parameters agreed with those obtained with the direct invasive method and can be utilized for easier and more convenient evaluation of sympathovagal activation.
These modulations appeared dramatically as a decrease in HR during the sleep onset (SO) period and progressively during NREM sleep to a minimum in SWS sleep. This shift of autonomic balance was similarly observed in subjects with obstructive sleep apnea (OSAS) and with various sleep disorders (VSD) maintaining relatively increased sympathetic tones over normal subjects [37].
Nonlinear measures also reflect the sympathovagal variations during the sleep stages. The most significant finding was the increase in the dominant chaoticity measured by a Lyapunov exponent and the decrease in degrees of freedom estimated by the correlation dimension during REM sleep compared to SWS [43]. The increase in dominant chaoticity during REM sleep is regarded as comparable to the increase in sympathetic tone, and the decrease in the correlation dimension might be an expression of the withdrawal of respiratory influences.
Instead of their separate use, interactions between heart and respiratory dynamics were investigated to differentiate physiological states and conditions according to sleep stages. The directionality of the coupling between respiratory and cardiac system reflects autonomic nervous activation and varies between sleep stages as well. The dominance of the coupling from the respiratory to the cardiac system was highest during wakefulness and REM sleep and decreased progressively toward bidirectional coupling as sleep moved to light sleep and SWS. The dominance of the coupling between the respiratory and cardiac systems also increased with the severity of OSA [44].
ECG-based sleep monitoring
Since heart rate changes as sleep progresses by sympathovagal modulation, the sleep stage at each epoch can be estimated by analyzing the heart rate dynamics of each epoch. If the performance of sleep stage scoring using heart rate is acceptable, sleep can be monitored as easily and conveniently as heart rate is measured, without using EEG.
ECG recorded with 3-directional acceleration using a patch-type device has used for the estimation of “Wake” and related sleep parameters. Wakefulness was determined based on information about movement from accelerometers and autonomic nervous activity estimated from ECG. Wake epochs were detected with an accuracy of 91.24% for healthy subjects and OSA patients compared to PSG results. Total sleep time (TST), sleep efficiency (SE), sleep latency (SL) and wake after sleep onset (WASO) were estimated with correlations higher than 0.75 compared to those determined from simultaneously recorded PSG [45].
For easier estimation of sleep efficiency without overnight recording, 5 min of recording of HRV with breathing during the waking period before sleep were analyzed and compared with the results obtained from the full night PSG recording. Since the sympathovagal activation level observed in the waking state before falling asleep has been associated with sleep, sleep efficiency was estimated via a multiple linear regression model with an absolute error of 2.18% and correlation of 0.94 compared to the PSG results [46].
The feasibility of sleep stage estimation using ECGs was investigated with the ECG recorded as one of PSG channels. An algorithm estimating SWS using only heart rates was developed to be utilized easily in mobile or wearable devices [47]. By quantifying autonomic stability using HR, they achieved an accuracy of 90.0% for SWS estimation for 21 healthy subjects and 24 OSA patients.
HRV parameters calculated from single-channel ECG R-peak (RR) intervals were applied to classify REM sleep. After 7 HRV parameters were determined to differentiate REM sleep from NREM sleep, principal component analysis and an adaptive threshold method were applied successively, and REM sleep classification was validated with an accuracy of 87% and kappa of 0.61 compared to the PSG results [48].
A single-lead ECG was used to investigate the feasibility of automatic classification of sleep stages and obstructive apneic epochs [49]. Instead of spectral features, median, inter-quartile range, and mean absolute deviation values simply calculated from RR intervals were chosen as features and applied for two-class classifications of each of the six sleep stages and to detect apneic epochs. Support vector machine (SVM) classified each sleep stage with a total accuracy of 73.1 and 76.9% for healthy and OSA patients, respectively.
The ECG channel was also investigated to differentiate subjects with a sleep-related breathing disorder from healthy subjects [50, 51]. When SVM was applied to wavelet decomposed sub-bands of HRV and the ECG derived respiration (EDR), an accuracy of 92.5% for diagnosing OSA having AHI > 10 was obtained [50].
AHI was estimated reliably without overnight recordings by a single channel RR interval recorded during 150 s of the sleep onset period. Variations of the respiration rate were calculated from RR interval data and applied as an input to AHI predictive model. The predictability of the model was validated with an absolute error of 3.65 events/h and correlation coefficient of 0.97 [52]. The method was elaborated by using RR interval data recorded in a 75 s experiment that consisted of a 60 s baseline measurement and consecutive 15 s guided deep inspiration breath-hold session. The predictive model estimated the AHI in a separate validation group of 92 subjects with an absolute error of 3.53 events/h and correlation coefficient of 0.99 relative to PSG results [53].
While the standard ECG recording is done with electrodes placed on the patient’s limbs or on the surface of the chest, new methods that make ECG recording less obtrusive are being developed. The ECG can be measured without direct contact of electrodes to the skin via capacitive coupling between the body surface and electrodes, and this method has been successfully applied for recording during sleep [54].
PPG-based sleep monitoring
Heart rate for the calculation of HRV can be measured by several methods. The electrocardiogram (ECG) is the basic method for heart rate monitoring and but heart rate can be recorded in several modes. Since photoplethysmograms (PPGs) and ballistocardiograms (BCGs) also represent the beating rhythm of the heart, they can be used for the calculation of HRV. Technological progress for unobtrusive measurements of these biological signals have made sleep monitoring convenient and possible in home environments.
PPGs are widely accepted as low cost, simple, and portable for use in smart watches and phones. Since a physiological link between cardiac activity, for example, indirectly measured with PPGs, and sleep is relatively well understood, sleep monitoring using PPGs has been greatly anticipated as an unobtrusive approach. Automatic sleep stage scoring based on HRV measured from wrist-worn PPGs combined with body movements measured with an accelerometer was compared to PSG and ACT [55]. This method discriminated sleep epochs from wake epochs with an accuracy of 91.5% and with a “Wake” detection sensitivity of 58.2%, which is significantly higher than the 45.5% of sensitivity of the ACT simultaneously used. The 3-class classification (Wake/NREM/REM) achieved an accuracy of 72.9%, and the 4-class classification (Wake/Light Sleep/SWS/REM) achieved an accuracy of 59.3%. The moderate epoch-by-epoch agreement and good agreement in terms of sleep statistics suggest that PPGs are promising for long-term sleep monitoring. Improvements in sleep/wake detection over ACT in a wrist-worn device can make PPGs complement PSGs in clinical practice.
A single-channel PPG has been used for the estimation of AHI. Respiration was derived from PPG baseline variation, envelope, and rate. This PPG-derived respiration (PDR) was further analyzed and processed by correlating to the saturation reduction. The pulse rate increases and pulse wave amplitude decreases during an apnea or hypopnea event, and the respiratory component of heart rate variability has a tendency to decrease before the apnea or hypopnea events [56]. AHI was estimated with a correlation of 0.81 for the subject group suspected of having OSA [57].
BCG-based sleep monitoring
A ballistocardiogram (BCG) measures the recoil force of the body in reaction to cardiac ejection of blood into the aorta and provides information on the timing of each heartbeat, like ECG and PPG. Since first observed in 1877, BCG has been developed in many forms and applications as an unobtrusive method for measuring heart rate and related cardiac functions [58].
BCG was measured unobtrusively by a load-cell-installed bed, and SWS was detected with an accuracy of 92.5% and kappa of 0.62 [59]. Among the HRV parameters, standard deviations of the RR intervals (SDNN), low frequency-to-high frequency ratio (LF/HF), alpha of detrended fluctuation analysis, and correlation coefficient of the RR interval were effective for SWS classification.
BCGs in combination with ECGs have been used to estimate arterial baroreflex activation by calculating the correlation between the RJ and RR intervals during an epoch [60]. Sleep latency (SL) was estimated with an error of 0.25 min based on the strong activation of arterial baroreflex after sleep onset.
BCG measured with PVDF film sensor on a bed mattress was used to detect nocturnal waking and estimating sleep efficiency effectively [61]. With an accuracy higher than 96.5% for awakening epoch detection, sleep efficiency was estimated with 1.05 and 1.44% error for healthy and OSA patients, respectively.
Based on the cardiorespiratory signals
If the dynamics of respiration in the sleep stages were combined with the information available from an ECG, estimation performance can be enhanced over that of ECG use only. An ECG combined with a respiratory effort signal were successfully used for the three-stage (Wake/NREM/REM) and four-stage (Wake/Light/Deep/REM) sleep estimation. After total of 142 features were extracted from the ECG and respiratory effort signal, features were sequentially modified, selected, and applied for sleep stage estimation [62]. They estimated three stages of sleep with accuracy of 80% and kappa coefficient of 0.56 and four stages of sleep with accuracy of 69% and kappa coefficient of 0.49. When the movement signal is added to cardiorespiratory signals, the performance can be enhanced, but the amount of enhancement is not significant. Studies using movement with cardiorespiratory signals produced similar accuracies of 81% and 69% but increased kappa coefficients of 0.62 and 0.56 for three and four stage sleep stage scoring, respectively [63].
Based on other types of signals
Peripheral arterial tonometry
Peripheral arterial tonometry (PAT), which measures pressure variations at fingertip under an applied sub-diastolic pressure, reflects sympathetic tone variations. Sixteen features extracted from PAT amplitudes and PAT-derived inter-pulse periods (IPP) in each interval were used for the prediction of REM sleep [64]. REM sleep epochs were predicted separately from the other stages of sleep with an accuracy of 89%. Two series of PAT amplitudes and PAT-derived IPP were also used to estimate light and deep sleep and achieved accuracy of 82% and 80%, respectively [65]. By integrating the movement signal from ACT with these signals, four stages of sleep can be estimated with an overall accuracy of 66% and kappa coefficient of 0.48 [66].
PAT has been suggested as an alternative indirect method to estimate AHI [67]. Each episode of sleep apnea is accompanied by a strong change in sympathetic activity, and this change was detected by combining flow decreases measured in PAT with an increase in HR as a marker for an apneic event and respiratory arousal [68]. One meta-study done over 13 studies reported an overall correlation of 0.89 between the respiratory disturbance index (RDI) from the PAT method and AHI from PSG [69].
EMG and EDA
An electromyogram (EMG) measured from the anterior tibialis muscles during PSG was investigated for its usability in sleep–wake classification [70]. Based on the reduced muscle tone during sleep, an accuracy higher than 80% was achieved with a specificity 71% higher than that of movement-based methods.
Electrodermal activity (EDA) refers to the variation in skin conductance controlled by sympathetic nervous activation and reflects physiological or psychological arousal. The EDA signal has been used to detect sleep onset and wake epochs during sleep, and the estimated performances were compared with ACT and PSG [71]. Sleep onset, offset, and total wake period were estimated with mean absolute errors of 4.1, 3.0, and 6.1 min, respectively, compared to PSG results. It outperformed ACT methods, which was attributed to the detection of long awakening periods that accompany physical quiescence and often missed in movement-based detection.
Summary
Sleep stage scoring and event detection are characterized by substantial uncertainty. When experts have rated the sleep stages using PSG, inter-rater reliabilities are reported between 82 and 89% range for five stage sleep scoring [72, 73]. This can be viewed as an apparent upper limit of performance for any simplified sleep scoring using fewer channels of biological signals. Table 2 summarizes the reported accuracies or correlations of the various methods.
Table 2.
Performances of reported sleep stage scoring and sleep parameter estimation
| SL | TST | WASO | N1 | N2 | N3 | REM | SE | 3CS | 4CS | AHI | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1ch. EEG | 85.01 | 22.01 | 85.01 | 86.01 | 95.01 | 96.0 | 92.1 | 84.9 | [11], [12]1 | |||
| Actigraphy | 0.84 | 86.0, 95.21 | [19], [25]1 | |||||||||
| Radar | 69.0 | 61.0 | 87.0 | 97.0 | 82.0 | 82.0 | [27] | |||||
| IR video | 92.0 | [28] | ||||||||||
| Respiration | 89.0 | 89.23 | 72.21 | 70.92 | 0.943 | [34], [35]1, [36]2, [26]3 | ||||||
| ECG | 0.81 | 0.75 | 0.78 | 98.54 | 62.04 | 90.01 | 87.02 | 91.2 | 92.53 | [45], [47]1, [48]2, [50]3, [49]4 | ||
| PPG | 91.5 | 72.9 | 59.3 | 0.811 | [55], [57]1 | |||||||
| BCG | 92.5 | 96.51 | [59], [61]1 | |||||||||
| ECG & respiration | 80.0 | 69.0 | [62] | |||||||||
| PAT | 80.0 | 89.01 | 66.02 | 0.893 | [64]1, [65], [66]2, [69]3 | |||||||
| Short term ECG | 0.94 | 0.991 | [46], [53]1 |
Numbers higher than 1.0 represent accuracies (%) and lower than 1.0 represent reported correlation coefficients
SL sleep latency, TST total sleep time, WASO wake after sleep onset, SE sleep efficiency, 3CS 3-class scoring, 4CS 4-class scoring, AHI apnea–hypopnea index
Single channel EEG exhibits the highest performance among the methods using a single-channel biological signal. This is because sleep is physiological state of the brain and it shares the same signal used for PSG for scoring. Sleep scoring methods based on movement are limited by sleep–wake discrimination and show low sensitivities in non-moving wake detection, which deteriorates the overall performances. In contrast to wearable ACT, movement sensors installed on bed showed significantly increased performance, which seems to be caused by the movement detected over the whole body rather than the simple movement of the wrist. Since respiration is accompanied by apparent movements, which can be monitored rather easily, it has used for smart sleep state scoring as well as breathing disorder detection. Various sensors are applied for unobtrusive monitoring of respiration and estimating sleep stages and AHI. Their performance for sleep–wake discrimination outperformed wearable ACT devices, and there have been several reports of successful results for three- and four-stage sleep scoring. Modulation of autonomic balance during sleep provided important clues for alternative and simpler sleep scoring methods based on cardiovascular activities, including ECG, PPG, BCG, and PAT, and many studies produced successful results. Since these types of signals can be easily integrated with wearable devices, they are anticipated to be used in applications for sleep monitoring in out of hospital environment. In contrast to the methods that record biological signals throughout the entire night, two studies used short term of ECG of 5 min and 75 s for SE and AHI estimation, respectively. The performances reported in these studies are very high for the studied populations. If sleep quality can be evaluated without sleep, it will be a breakthrough for sleep studies and extend the methods for the enhancement of sleep quality.
Conclusion
As sleep occupies one-third of our total life, maintaining good sleep quality is very important for wellness. Good sleep quality also enhances the quality of daytime activities. Maintaining good sleep quality begins with sleep monitoring. Sleep monitoring, which used to be done by PSG in specialized clinics, is now possible in many simplified and innovative ways in home environments. Sleep can be monitored unobtrusively without interrupting our ordinary sleep pattern. With continual daily monitoring of sleep, we can evaluate daily sleep quality, diagnose sleep related disorders, and detect comorbid chronic or mental diseases in earlier stages. Quantitative evaluation of sleep is the starting point for environmental conditioning and sleep modulation to achieve sleep quality enhancement and mitigate the related symptoms and diseases. Smarter technologies for sleep monitoring at home will promote sleep from the current passive stage to a future active stage to enhance our quality of life.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2017R1A5A1015596) and Samsung Electronics (800-20180337) Co. Ltd.
Conflict of interest
All authors declare to have no conflict of interests.
Ethical approval
All procedures performed in studies involving human participants were approved by the Institutional Review Board of Seoul National University Hospital, Korea.
Informed consent
Informed consent was obtained from all individual participants included.
Contributor Information
Kwang Suk Park, Email: pks@bmsil.snu.ac.kr.
Sang Ho Choi, Email: csh412@snu.ac.kr.
References
- 1.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of Type 2 diabetes. Diabetes Care. 2006;29(3):657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 2.Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, Rundle AG, Zammit GK, Malaspina D. Short sleep duration as a risk factor for hypertension. Hypertension. 2006;47(5):833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 3.Perlman CA, Johnson SL, Mellman TA. The prospective impact of sleep duration on depression and mania. Bipolar Disord. 2006;8(3):271–274. doi: 10.1111/j.1399-5618.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- 4.Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31(5):619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deak M, Epstein LJ. The history of polysomnography. Sleep Med Clin. 2009;4(3):313–321. [Google Scholar]
- 6.Berry RB, Albertario CL, Harding SM, Lloyd RM, Plante DT, Quan SF, Troester MM, Vaughn BV. The AASM manual for the scoring of sleep and associated events. In: American Academy of Sleep Medicine. 2018; version 2.5.
- 7.Van de Water ATM, Holmes A, Hurley DA Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography-a systematic review. J Sleep Res. 2011;20(1):183–200. doi: 10.1111/j.1365-2869.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 8.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, Hudgel D, Sateia M, Schwab R. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients portable monitoring. J Clin Sleep Med. 2007;3(7):737–752. [PMC free article] [PubMed] [Google Scholar]
- 9.Mykytyn IJ, Sajkov D, Neil AM, McEvoy RD. Portable computerized polysomnography in attended and unattended settings. Chest. 1999;15(1):114–122. doi: 10.1378/chest.115.1.114. [DOI] [PubMed] [Google Scholar]
- 10.Bruyneel M, Sanida C, Art G, Libert W, Cuveler L, Paesmans M, Sergysels R, Ninane V. Sleep efficiency during sleep studies: results of a prospective study comparing home-based and in-hospital polysomnography. J Sleep Res. 2011;20(1):201–206. doi: 10.1111/j.1365-2869.2010.00859.x. [DOI] [PubMed] [Google Scholar]
- 11.Berthomier C, Drouot X, Herman-Stoïca M, Berthomier P, Prado J, Bokar-Thire D, Benoit O, Mattout J, d’Ortho MP. Automatic analysis of single-channel sleep EEG: validation in healthy individuals. Sleep. 2007;30(11):1587–1595. doi: 10.1093/sleep/30.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang SF, Kuo CE, Hu YH, Pan YH. Automatic stage scoring of single channel sleep EEG by using multiscale entropy and autoregressive models. IEEE T Instrum Meas. 2012;61(6):1649–1657. [Google Scholar]
- 13.Shambroom JR, Fabregas SE, Johnstone J. Validation of an automated wireless system to monitor sleep in healthy adults. J Sleep Res. 2012;21(2):221–230. doi: 10.1111/j.1365-2869.2011.00944.x. [DOI] [PubMed] [Google Scholar]
- 14.Stochholm A, Mikkelsen K, Kidmose P. Automatic sleep stage classification using ear EEG. In: Conference proceedings of the IEEE EMBC. 2016. 10.1109/embc.2016.7591789. [DOI] [PubMed]
- 15.Association American Sleep Disorder. Practice parameters for the use of actigraphy in the clinical assessment of sleep disorders. Sleep. 1995;18(4):285–287. doi: 10.1093/sleep/18.4.285. [DOI] [PubMed] [Google Scholar]
- 16.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med. 2002;6(2):113–124. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- 17.Morgenthaler T, Alessi C, Friedman L, Owens J, Kapur V, Boehlecke B, Brown T, Chesson A, Jr, Coleman J, Lee-Chiong T, Pancer J, Swick TJ, Standards of Practice Committee. American Academy of Sleep Medicine Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep. 2007;30(4):519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 18.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med. 2011;15:259–267. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Marino M, Rueschman MN, Winkelman JW, Ellenbogen JM, Solet JM, Dulin H, Berkman LF, Buxton OM. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755. doi: 10.5665/sleep.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly JM, Strecker RE, Bianchi MT. Recent developments in home sleep-monitoring devices. ISRN Neurol. 2012. 10.5402/2012/768794. [DOI] [PMC free article] [PubMed]
- 21.Fino E, Mazzetti M. Monitoring healthy and disturbed sleep through smartphone applications: a review of experimental evidence. Sleep Breath. 2018. 10.1007/s1132501816613. [DOI] [PubMed]
- 22.Yang CC, Hsu YL. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors. 2010;10:7772–7788. doi: 10.3390/s100807772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko PR, Kientz JA, Choe EK, Kay M, Landis CA, Watson NF. Consumer sleep technologies: a review of the landscape. J Clin Sleep Med. 2015;11(12):1455–1461. doi: 10.5664/jcsm.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantua J, Gravel N, Spencer RMC. Reliability of sleep measures from four personal health monitoring devices compared to research-based actigraphy and polysomnography. Sensors. 2016;16:646. doi: 10.3390/s16050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi BH, Seo JW, Choi JM, Shin HB, Lee JY, Jeong DU, Park KS. Non-constraining sleep/wake monitoring system using bed actigraphy. Med Biol Eng Comp. 2007;45(1):107–114. doi: 10.1007/s11517-006-0134-1. [DOI] [PubMed] [Google Scholar]
- 26.Hwang SW, Lee YJ, Jeong DU, Park KS. Unconstrained sleep stage estimation based on respiratory dynamics and body movement. Methods Info Med. 2016;55(06):545–555. doi: 10.3414/ME15-01-0140. [DOI] [PubMed] [Google Scholar]
- 27.De Chazal P, O’Hare E, Fox N, Heneghan C. Assessment of sleep/wake patterns using a non-contact biomotion sensor. In: Conference proceedings of the IEEE engineering in medicine and biology society. 2008. p. 514–7. [DOI] [PubMed]
- 28.Liao WH, Yang CM. Video-based activity and movement pattern analysis in overnight sleep studies. In: Conference proceedings of the IEEE pattern recognition. 2008. 10.1109/icpr.2008.4761635.
- 29.Nakatani M, Okada S, Shimizu S, Mohri I, Ohno Y, Taniike M, Makikawa M. Body movement analysis during sleep for children with ADHD using video image processing. In: Conference proceedings of the IEEE engineering in medicine and biology society. 2013. p. 6389–92. [DOI] [PubMed]
- 30.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malik V, Smith D, Lee-Chiong T., Jr Respiratory physiology during sleep. Sleep Med Clin. 2012;5(2):497–505. [Google Scholar]
- 32.Douglas NJ, White DP, Pickett CK, Weil JV, Zwillich CW. Respiration during sleep in normal man. Thorax. 1982;37(11):840–844. doi: 10.1136/thx.37.11.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward KL, McArdle N, James A, Bremner AP, Simpson L, Cooper MN, Palmer LJ, Fedson AC, Mukherjee S, Hillman DR. A comprehensive evaluation of a two-channel portable monitor to “rule in” obstructive sleep apnea. J Clin Sleep Med. 2015;11(4):433–444. doi: 10.5664/jcsm.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung GS, Choi BH, Lee JS, Lee JS, Jeong DU, Park KS. REM sleep estimation only using respiratory dynamics. Physiol Meas. 2009;30(12):1327–1340. doi: 10.1088/0967-3334/30/12/003. [DOI] [PubMed] [Google Scholar]
- 35.Samy L, Huang MC, Liu JJ, Xu W, Sarrafzadeh M. Unobtrusive sleep stage identification using a pressure-sensitive bed sheet. IEEE Sens J. 2014;14(7):2091–2101. [Google Scholar]
- 36.Hwang SH, Lee HJ, Yoon HN, Jung DW, Lee JG, Lee YJ, Jeong DU, Park KS. Unconstrained sleep apnea monitoring using polyvinylidene fluoride film-based sensor. IEEE Trans Biomed Eng. 2014;61(7):2125–2134. doi: 10.1109/TBME.2014.2314452. [DOI] [PubMed] [Google Scholar]
- 37.Shinara Z, Akselroda S, Daganb Y, Baharava A. Autonomic changes during wake–sleep transition: a heart rate variability based approach. Auton Neurosci. 2006;130(1):17–27. doi: 10.1016/j.autneu.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 39.Baharav A, Kotagal S, Gibbons V, Rubin BK, Pratt G, Karin J, Akselrod S. Fluctuations in autonomic nervous activity during sleep displayed by power spectrum analysis of heart rate variability. Neurology. 1995;45(6):1183–1187. doi: 10.1212/wnl.45.6.1183. [DOI] [PubMed] [Google Scholar]
- 40.Voronin IM, Biryukova EV. Heart rate variability in healthy humans during night sleep. Hum Physiol. 2006;32(3):258–263. [PubMed] [Google Scholar]
- 41.Verrier RL, Harper RM, Hobson JA. Cardiovascular physiology: central and autonomic regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Philadelphia: Saunders; 2000. pp. 179–191. [Google Scholar]
- 42.Stein PK, Pu Y. Heart rate variability, sleep and sleep disorders. Sleep Med Rev. 2012;16(1):47–66. doi: 10.1016/j.smrv.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Fell J, Mann K, RoÈschke J, Gopinathan MS. Nonlinear analysis of continuous ECG during sleep II. Dynamical measures. Biol Cybern. 2000;82(6):485–491. doi: 10.1007/s004220050601. [DOI] [PubMed] [Google Scholar]
- 44.Yoon HN, Choi SH, Kwon HB, Kim SK, Hwang SH, Oh SM, Choi JW, Lee YJ, Jeong DU, Park KS. Sleep-dependent directional coupling of cardiorespiratory system in patients with obstructive sleep apnea. In: IEEE transactions on biomedical engineering. 2018. 10.1109/tbme.2018.2819719. [DOI] [PubMed]
- 45.Yoon HN, Hwang SH, Choi SH, Choi JW, Lee YJ, Jeong DU, Park KS. Wakefulness evaluation during sleep for healthy subjects and OSA patients using a patch-type device. Comput Methods Prog Biomed. 2018;155:127–138. doi: 10.1016/j.cmpb.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Jung DW, Lee YJ, Jeong DU, Park KS. New predictors of sleep efficiency. Chronobiol Int. 2017;34(1):93–104. doi: 10.1080/07420528.2016.1241802. [DOI] [PubMed] [Google Scholar]
- 47.Yoon HN, Hwang SH, Choi JW, Lee YJ, Jeong DU, Park KS. Slow-wave sleep estimation for healthy subjects and OSA patients using R–R intervals. IEEE J Biomed Health Inform. 2018;22(1):119–128. doi: 10.1109/JBHI.2017.2712861. [DOI] [PubMed] [Google Scholar]
- 48.Yoon HN, Hwang SH, Choi JW, Lee YJ, Jeong DU, Park KS. REM sleep estimation based on autonomic dynamics using R–R intervals. Physiol Meas. 2017;38(4):631–651. doi: 10.1088/1361-6579/aa63c9. [DOI] [PubMed] [Google Scholar]
- 49.Yılmaz B, Asyalı MH, Arıkan E, Yetkin S, Özgen F. Sleep stage and obstructive apneaic epoch classification using single-lead ECG. BioMed Eng Online. 2010;9:39. doi: 10.1186/1475-925X-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khandoker AH, Palaniswami M, Karmakar CK. Support vector machines for automated recognition of obstructive sleep apnea syndrome from ECG recordings. IEEE Trans Info Technol Biomed. 2009;13(1):37–48. doi: 10.1109/TITB.2008.2004495. [DOI] [PubMed] [Google Scholar]
- 51.Bsoul M, Minn H, Tamil L. Apnea MedAssist: real-time sleep apnea monitor using single-lead ECG. IEEE Trans Info Technol Biomed. 2011;15(3):416–427. doi: 10.1109/TITB.2010.2087386. [DOI] [PubMed] [Google Scholar]
- 52.Jung DW, Hwang SH, Lee YJ, Jeong DU, Park KS. Apnea–hypopnea index prediction using electrocardiogram acquired during sleep-onset period. IEEE Trans Biomed Eng. 2017;64(2):295–301. doi: 10.1109/TBME.2016.2554138. [DOI] [PubMed] [Google Scholar]
- 53.Jung DW, Lee YJ, Jeong DU, Park KS. Apnea–hypopnea index prediction through an assessment of autonomic influence on heart rate in wakefulness. Physiol Behav. 2017;169:9–15. doi: 10.1016/j.physbeh.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Lim YG, Kim KK, Park KS. ECG recording on a bed during sleep without direct skin-contact. IEEE Trans Biomed Eng. 2007;54(4):718–725. doi: 10.1109/TBME.2006.889194. [DOI] [PubMed] [Google Scholar]
- 55.Fonseca P, Weysen T, Goelema MS, Møst EIS, Radha M, Scheurleer CL, van den Heuvel L, Aarts RM. Validation of photoplethysmography-based sleep staging compared with polysomnography in healthy middle-aged adults. Sleep. 2017;40(7):zsx097. doi: 10.1093/sleep/zsx097. [DOI] [PubMed] [Google Scholar]
- 56.Gil E, Mendez M, Vergara JM, Cerutti S, Bianchi AM, Laguna P. Discrimination of sleep-apnea–related decreases in the amplitude fluctuations of PPG signal in children by HRV analysis. IEEE Trans Biomed Eng. 2009;56(4):1005–1014. doi: 10.1109/TBME.2008.2009340. [DOI] [PubMed] [Google Scholar]
- 57.Romem A, Romem A, Koldobskiy D, Scharf SM. Diagnosis of obstructive sleep apnea using pulse oximeter derived photoplethysmographic signals. J Clin Sleep Med. 2014;10(3):285–290. doi: 10.5664/jcsm.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inan OT, Migeotte PF, Park KS, Etemadi M, Tavakolian K, Casanella R, Zanetti J, Tank J, Funtova I, Prisk GK, Rienzo MD. Ballistocardiography and seismocardiography: a review of recent advances. IEEE J Biomed Health Inform. 2015;19(4):1414–1427. doi: 10.1109/JBHI.2014.2361732. [DOI] [PubMed] [Google Scholar]
- 59.Choi BH, Chung GS, Lee JS, Jeong DU, Park KS. Slow-wave sleep estimation on a load-cell-installed bed: a non-constrained method. Physiol Meas. 2009;30(11):1163–1170. doi: 10.1088/0967-3334/30/11/002. [DOI] [PubMed] [Google Scholar]
- 60.Jung DW, Hwang SH, Chung GS, Lee YJ, Jeong DU, Park KS. Estimation of sleep onset latency based on the blood pressure regulatory reflex mechanism. IEEE J Biomed Health Inform. 2013;17(3):539–544. doi: 10.1109/jbhi.2013.2257816. [DOI] [PubMed] [Google Scholar]
- 61.Jung DW, Hwang SH, Yoon HN, Lee YJG, Jeong DU, Park KS. Nocturnal awakening and sleep efficiency estimation using unobtrusively measured ballistocardiogram. IEEE Trans Biomed Eng. 2014;61(1):131–138. doi: 10.1109/TBME.2013.2278020. [DOI] [PubMed] [Google Scholar]
- 62.Fonseca P, Long X, Radha M, Haakma R, Aarts RM, Rolink J. Sleep stage classification with ECG and respiratory effort. Physiol Meas. 2015;36(10):2027–2040. doi: 10.1088/0967-3334/36/10/2027. [DOI] [PubMed] [Google Scholar]
- 63.Willemen T, Van Deun D, Verhaert V, Vandekerckhove M, Exadaktylos V, Verbraecken J, Van Huffel S, Haex B, Sloten JV. An evaluation of cardiorespiratory and movement features with respect to sleep-stage classification. IEEE J Biomed Health Inform. 2014;18(2):661–669. doi: 10.1109/JBHI.2013.2276083. [DOI] [PubMed] [Google Scholar]
- 64.Herscovici S, Pe’er A, Papyan S, Lavie P. Detecting REM sleep from the finger: an automatic REM sleep algorithm based on peripheral arterial tone (PAT) and actigraphy. Physiol Meas. 2007;28(2):129–140. doi: 10.1088/0967-3334/28/2/002. [DOI] [PubMed] [Google Scholar]
- 65.Bresler M, Sheffy K, Pillar G, Preiszler M, Herscovici S. Differentiating between light and deep sleep stages using an ambulatory device based on peripheral arterial tonometry. Physiol Meas. 2008;29(5):571–584. doi: 10.1088/0967-3334/29/5/004. [DOI] [PubMed] [Google Scholar]
- 66.Hedner J, White DP, Malhotra A, Herscovici S, Pittman SD, Zou D, Grote L, Pillar G. Sleep staging based on autonomic signals: a multi-center validation study. J Clin Sleep Med. 2011;7(3):301–306. doi: 10.5664/JCSM.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schnall RP, Shlitner A, Sheffy J, Kedar R, Lavie P. Periodic, profound peripheral vasoconstriction—a new marker of obstructive sleep apnea. Sleep. 1999;22:939–946. [PubMed] [Google Scholar]
- 68.Penzel T, Kesper K, Pinnow I, Becker HF, Vogelmeier C. Physiol Meas. 2004;25(4):1025–1036. doi: 10.1088/0967-3334/25/4/019. [DOI] [PubMed] [Google Scholar]
- 69.Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;39(12):1343–1350. doi: 10.1001/jamaoto.2013.5338. [DOI] [PubMed] [Google Scholar]
- 70.Hwang SH, Chung GS, Lee JS, Shin JH, Lee SJ, Jeong DU, Park KS. Sleep-wake estimation using only anterior tibialis electromyography data. Biomed Eng Online. 2012;11:26. doi: 10.1186/1475-925X-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hwang SH, Seo SW, Yoon HN, Jung DW, Baek HJ, Cho JG, Choi JW, Lee YJ, Jeong DU, Park KS. Sleep period time estimation based on electrodermal activity. IEEE J Biomed Health Inform. 2017;21(1):115–122. doi: 10.1109/JBHI.2015.2490480. [DOI] [PubMed] [Google Scholar]
- 72.Danker-Hopfe H, Anderer P, Zeitlhofer J, Boeck M, Dorn H, Gruger G, Heller E, Loretz E, Moser D, Parapatics S, Saletu B, Schmit A, Dorffner G. Interrater reliability for sleep scoring according to Rechtschaffen & Kales and the new AASM standard. J Sleep Res. 2009;18(1):78–84. doi: 10.1111/j.1365-2869.2008.00700.x. [DOI] [PubMed] [Google Scholar]
- 73.Nonoue S, Mashita M, Haraki S, Mikami A, Adachi H, Yatani H, Yoshida A, Taniike M, Kato T. Inter-scorer reliability of sleep assessment using EEG and EOG recording system in comparison to polysomnography. Sleep Biol Rhythms. 2017;15(1):39–48. [Google Scholar]