Abstract
Objective:
Obesity during pregnancy impedes fetal iron endowment. In adults, both iron depletion and hypoxia stimulate erythropoietin (Epo) production, while hepcidin, the primary iron regulator, is inhibited by Epo and stimulated by obesity. To understand this relationship in fetuses, we investigated obesity, inflammation, and fetal iron status on fetal Epo and hepcidin levels.
Study Design:
Epo, hepcidin, C-reactive protein (CRP), and ferritin levels were measured in 201 newborns of 35–40 weeks gestation with historical risk factors for a low fetal iron endowment, including half with maternal obesity.
Results:
Epo was unrelated to fetal size, but Epo was directly related to maternal BMI (kg/m2) (p <0.03) and CRP (p <0.0005) at delivery. Epo levels were twice as likely to be elevated (≥50 IU.L) comparing the lowest quartile of ferritin to the upper three quartiles (p<0.01). Hepcidin was directly related to ferritin (p<0.001), and indirectly related to maternal BMI (p <0.015), but BMI became nonsignificant when undergoing multivariate analysis. Hepcidin was unrelated to Epo.
Conclusion:
Although some of the fetal responses involving Epo were similar to adults, we did not find a hepcidin-Epo relationship like that of adults, where the liver is the site of both hepcidin and Epo production.
Keywords: erythropoietin, iron, maternal obesity, hepcidin, ferritin, C-reactive protein, large for gestational age
INTRODUCTION
One-third of women of childbearing age in the U.S. are obese, a major risk factor for poor perinatal outcomes.1 Maternal obesity, either alone or with diabetes mellitus, stimulates fetal overgrowth and increases fetal demand of nutrients including iron.1,2 Our research3 and that of Jones et al.4 found that maternal obesity or excessive gestational weight gain impeded fetal iron endowment. This may be due in part to obesity related inflammation, which is known to inhibit the required pregnancy-induced increase in enteral iron absorption.5 Nearly half of the iron required to fulfill growth-related needs during the first year of life should be acquired before birth.7 Developing iron deficiency (ID) as a fetus or infant can disrupt brain development and cause long-term cognitive deficits.6 Thus, understanding the physiology of fetal iron regulators, erythropoietin (Epo) and hepcidin, in obesity is of the utmost importance.
Epo, the primary regulator of erythropoiesis, is upregulated in fetuses from diabetic pregnancies due to the fetal overgrowth induced hypoxia.7,8 In the adult, Epo is produced primarily in the kidney; in the fetus, Epo is produced primarily in the liver.9 Hypoxia upregulates Epo in both fetuses and adults,10 while iron as a regulator of Epo has only been studied in adults.11 The fetal liver accrues iron, but it is not known whether ID would stimulate fetal liver Epo production. Because maternal plasma Epo does not cross the placenta, fetal levels of Epo, and its effects would reflect fetal physiology. One prior study found higher fetal erythropoiesis during obese pregnancies12 and another higher fetal Epo and lower ferritin levels in obese pregnancies.13 However, the physiological mechanisms underlying these complex relationships are yet to be explored.
Hepcidin is the recently discovered master iron regulator. Hepcidin binds to ferroportin on the basal enterocyte and macrophage membrane, degrading ferroportin and blocking iron absorption.14 Normally, maternal hepcidin levels fall during pregnancy in order to increase maternal dietary iron absorption and promote active transport of iron across the placenta.15,16,17 However, hepcidin overexpression persists in obese pregnancies due to low-grade chronic inflammation.16,17 In adults, Epo administration results in erythroblast production of erythroferrone, which suppresses hepcidin levels,18,19 but this has not been studied in fetuses. Thus, it is not known whether fetal hepcidin levels would be higher due to maternal obesity-related inflammatory activity or lower due to fetal ID-induced Epo production.
The current paper extends our previous work3 to investigate the relationship between fetal Epo and fetal hepcidin during altered iron biology in obese pregnancies. The objective was to examined maternal obesity, inflammation, and iron status on fetal Epo and fetal hepcidin within a healthy birth cohort. Because of our interest in iron, we recruited known demographic and medical risk factors for developing infantile ID. We hypothesized that iron-challenged fetuses born to women with obesity would exhibit higher Epo and lower hepcidin levels.
MATERIALS AND METHODS
Subjects
This prospective observational birth cohort study was approved by the Institutional Review Boards of the University of Wisconsin and UnityPoint Meriter, Madison, WI. Cord blood was collected prospectively at delivery. After delivery, but before hospital discharge, mothers and newborns were recruited and written informed consent obtained. Mothers of healthy singleton or twin term or late preterm newborns ≥35 weeks’ gestation between the ages of 18–40 years were screened for eligibility using daily birth logs and patient records at the Birthing Center at UnityPoint Health Meriter. Medical records were screened for known demographic and medical risk factors for infantile ID.20 Previous data analyses indicated that obesity was an additional risk factor beyond previously established demographic and clinical factors.3 Timing of clamping of the umbilical cord was performed at the discretion of the delivering provider and recorded. Subjects were excluded for HIV positive status, fetal anemia, NICU admission, or not being discharged with their mother. Both English-speaking and Spanish-speaking mothers were eligible. After informed consent, chart reviews of maternal anthropometric data were used to determine BMI before pregnancy and at delivery.
Sample Collection and Processing.
The Birthing Center collected umbilical cord blood at delivery and stored it at 4 °C. Within 8 days of collection,20 blood was assayed for hemoglobin (Hb) by pocH-100i hematology analyzer (Sysmex, Mundelein, IL, USA) and erythrocyte zinc protoporphyrin/heme (ZnPP/H) iron by hematofluorometry (Aviv Biomedical, Lakewood, NJ, USA) after washing.21 Reticulocyte (RE)-ZnPP/H was measured using the most immature fraction of erythrocytes to improve ZnPP/H sensitivity,21 analogous to reticulocyte Hb. Cord blood was centrifuged at 1000 g for 4 min with plasma stored at −80 oC until assay. Ferritin (Bio-Quant, San Diego, CA, USA), high sensitivity hepcidin (DRG International, Springfield, NJ, USA), high-sensitivity CRP (Bio-Quant, Inc., San Diego, CA, USA) were measured in plasma using commercial enzyme-linked immunosorbent kits. This smaller cohort was selected for availabile plasma samples for determination of Epo (Quantikine IVD ELISA, R&D Systems, Minneapolis, MN, USA).
Data Analysis
Maternal morphometric measures were used to determine BMI at conception and at delivery, and set, a priori, the nongravid definition to designate obesity (BMI ≥30 kg/m2). The lean comparison group was defined as all others with BMI <30 kg/m2. Excessive pregnancy weight gain was ≥18 kg (40 lb), the maximal amount recommended for any weight group following the 2009 American College of Obstetricians and Gynecologists guidelines.22 Neonatal Z-scores for weight were determined by birth weight, gender, and gestational age.7 LGA was defined by Z-score of >2 and SGA as <−2. ΔZnPP/H was the difference between washed and RE-ZnPP/H.21 Before the study began, based on previous data from our laboratory, the 75th percentile for ZnPP/H was set at 95 μmol/mol where higher ZnPP/H indicates greater ID and the 25th percentile for serum fetal ferritin was 84 ng/mL.3,20 The literature reported an elevated fetal Epo at ≥50 U/L.23 The distributions of variables were analyzed, with ln-conversion of Epo, ZnPP/H, and ferritin to normalize distribution. Fisher’s exact or χ2 tests examined nominal data; unpaired t-tests for normal and natural log conversion was performed to normalize data for Epo, ZnPP/H, ferritin, and CRP. In addition, simple and stepwise regression, as well as logistic regression methods were used. A p-value of <0.05 determined significance; data in figures are illustrated as Means ± SEM.
RESULTS
Demographic Characteristics.
This 201 member cohort was selected from a larger 316-member cohort3 because of the availability of fetal Epo determination. Enrollees were healthy without medical complications, but recruitment strategy targeted one or more demographic or medical risks for their infants becoming ID during the first year of life, with a mean of 3.4 risks. Maternal diagnoses included anemia and gestational diabetes in some cases, but all neonates were clinically healthy between 35 and 41 weeks gestation. The majority of families reported being Caucasian (N=157); 54 families reported minority status. Eleven women were prescribed additional iron supplements for anemia diagnosed early in prenatal care.
Clinical data were analyzed with respect to maternal obesity measured at delivery, Table 1. Women with obesity were more likely to be obese at conception, diagnosed with diabetes during pregnancy, and experienced cesarean delivery, while newborns were larger and a greater percent were LGA, χ2 tests, Table 1. Ethnic distribution of the women did not differ.
Table 1:
Demographic and morphometric parameters shown with respect to maternal obesity at delivery (BMI < or > 30 kg/m2).
| Lean at Delivery (N = 96) |
BMI≥30 kg/m2 @ Delivery (N =105) |
P value | |
|---|---|---|---|
| Maternal BMI at Conception (kg/m2) | 22.0 ± 0.2 | 31.4 ± 0.5 | <0.0001 |
| Maternal BMI at Delivery (kg/m2) | 26.7 ± 0.2 | 36.8 ± 0.4 | <0.0001 |
| Cesarean section (%) | 17.1 | 30.6 | 0.008 |
| Maternal Diabetes (%) | 23.2 | 35.8 | 0.035 |
| Maternal IDA beginning (%) | 36.8 | 39.6 | 0.398 |
| Gestational Age (weeks) | 39.2 ± 0.1 | 39.3 ±0.1 | 0.922 |
| Gestational Age 35–37 Weeks (%) | 3.2% | 3.8% | 0.812 |
| Birth Weight (kg) | 3.45 ± 0.06 | 3.75 ± 0.06 | 0.0002 |
| Birth Weight Z-score | –0.10 ± 0.17 | 0.74 ± 0.14 | 0.001 |
| LGA-Newborn (%) | 25.2 | 41.5 | 0.011 |
| Immediate Cord Clamping (%) | 72.6 | 83.0% | 0.108 |
Abbreviations: BMI=body mass index, IDA=iron deficiency anemia, LGA=large-for-gestation.
Hematopoietic Parameters.
Epo was higher, hepcidin was lower, Hb was higher, ZnPP/H in the immature reticulocyte fraction (RE-ZnPP/H) was higher, and ferritin was lower in the obese at delivery group as compared to the lean group, t-tests, Table 2.
Table 2.
Fetal Hematological parameters shown with respect to maternal obesity at delivery (BMI < or > 30 kg/m2).
| Hematological | Lean at Delivery (N = 96) |
BMI≥30 kg/m2 @ Delivery (N =105) |
P value |
|---|---|---|---|
| Epo (U/L) | 35.5 ± 4.8 | 44.5 ± 4.9 | 0.008 |
| Epo >50 U/L (%) | 18.8 | 23.6 | 0.253 |
| Hepcidin (ng/mL) | 30.3 ± 1.9 | 23.6 ± 2.2 | <0.0001 |
| Hemoglobin (g/L) | 159 ± 2 | 166 ± 2 | 0.025 |
| Hemoglobin >75th Percentile (%) | 11.5 | 12.2 | 0.023 |
| ZnPP/H (μmol/mol) | 94.2 ± 2.6 | 100.1 ± 2.9 | 0.090 |
| ZnPP/H >75th percentile (%) | 41.9 | 42.9 | 0.481 |
| ReZnPP/H (μmol/mol) | 115.4 ± 3.7 | 126.6 ± 3.6 | 0.013 |
| Ferritin (ng/mL) | 168.2 ± 9.5 | 137.7 ± 7.5 | 0.002 |
| Ferritin <25th (%) | 17.6 | 27.2 | 0.035 |
| CRP (mg/dL) | 0.252 ± 0.06 | 0.254 ± 0.08 | 0.229 |
Epo=erythropoietin, ZnPP/H=zinc protoporphyrin/heme, ReZnPP/H=reticulocyte ZnPP/H (youngest erythrocytes), CRP=C-reactive protein.
Relationships between Epo, Growth and Iron.
We found that neither fetal birth weight nor neonatal BMI was related to Epo levels by regression analyses. However, by regression. maternal BMI at delivery was directly related to Epo, Fig. 1A. A transition from being relatively lean at conception to being obese at delivery was also associated with higher Epo levels (44.5 ± 4.9 in obesity vs. 35.5 ± 4.8 IU/L in lean, p=0.010). Epo was not related to mode of delivery, sex, or race/ethnicity background.
Figure 1. Maternal Body Mass Index (BMI), Fetal Ferritin and Fetal Epo.
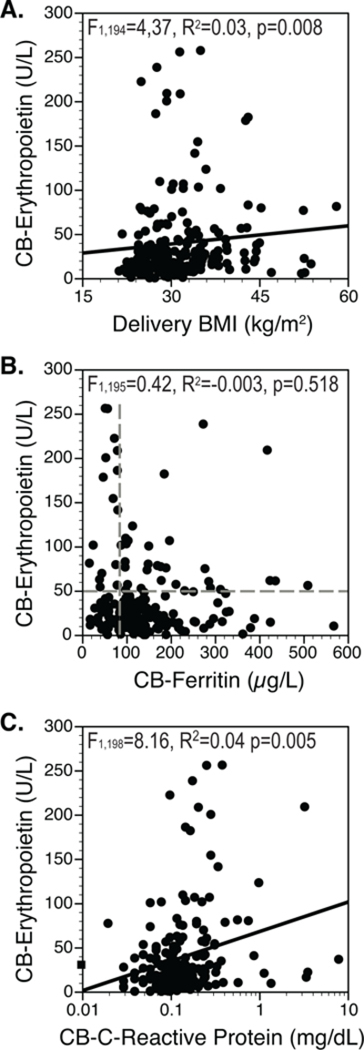
A. Fetal cord blood (CB) Epo was directly related to maternal BMI at delivery. B. CB-Epo was not related to ferritin across the entire range, F1,195=0.42, p=0.518. When the lowest quartile of ferritin (ID) was demarcated by vertical dashed line, compared to the iron sufficient (IS), proportionately more Epo levels in the ID group were elevated at ≥50 IU/L, shown in the figure by the horizontal dashed line, (n=16/44 of ID, 36.3% vs. n=27 of 153 of IS, 17.5%, p=0.009). C. CB-Epo was directly related to CRP.
Epo and ferritin were not related by regression analyses, but a linkage was evident when analyzed in a dichotomous manner, Fig. 1B. Mean Epo was higher if ferritin was below the 25th percentile (ID group) as compared to the upper 75th percentile (67.2 U/L vs. 33.3 U/L, p=0.002). The vertical and horizontal dashed lines in Fig. 1B show by χ2 testing that there were proportionately more elevated Epo values (≥50 IU/L) in the lowest quartile of ferritin (ID). In addition, by regression Epo levels were directly related to CRP, Fig. 1C.
Using stepwise regression analysis to include maternal delivery BMI, CRP and ferritin in the statistical model, only CRP predicted Epo levels (F1,195=7.5, R2=0.04, p=0.007). Examining the lowest quartile of ferritin on Epo in a multivariate logistic regression model, both low ferritin (F2,197=17.2, R2=0.08, p=0.001) and CRP (F2,197=8.12, R2=0.04, p=0.005) was directly associated with Epo.
Relationships between Hepcidin, Epo, Fetal Growth, and Iron.
By regression analysis, hepcidin was not related to Epo levels, birth weights, or birth weight Z-scores, though it was inversely related to maternal BMI. Fig. 2A. Hepcidin was also significantly related to ferritin levels Fig. 2B. Hepcidin was lower in neonates with low ferritin (ID group) as compared to those in the upper 3 quartiles (17.1 ± 2.4 vs. 29.7 ± 1.7, p<0.0001). A stepwise regression model including ferritin and maternal BMI at delivery indicated that hepcidin was more significantly predicted by ferritin (p<0.0001) than by BMI at delivery (p=0.001). Hepcidin levels were not related to CRP, Fig. 2C.
Figure 2. Maternal BMI, Fetal Ferritin and Fetal Hepcidin.
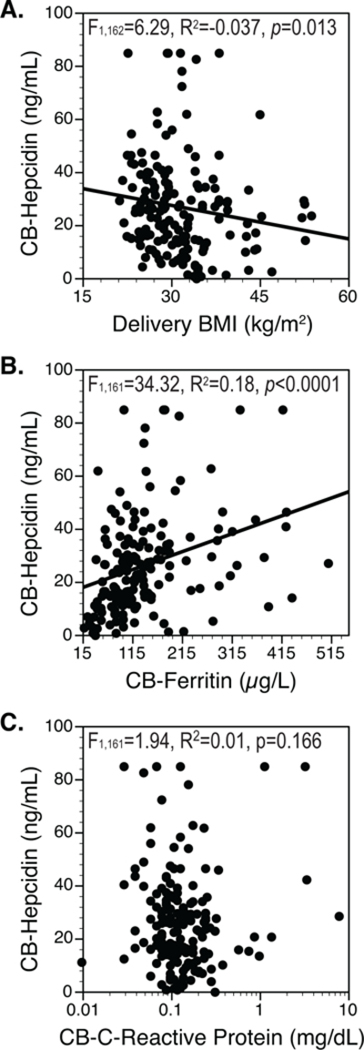
A. Fetal (CB) hepcidin was indirectly related to maternal BMI (kg/m2) at delivery. B. CB-Hepcidin was also directly related to ferritin. C. However, CB-hepcidin was not related to CRP.
DISCUSSION
This study confirms and extends previous reports indicating that there is a complex interplay between maternal obesity during gestation, fetal growth patterns, and inflammatory physiology, which can impede fetal iron transfer and thus impact Epo and hepcidin levels. By regression analyses, Epo was directly and hepcidin indirectly related to maternal BMI, despite the fact that neither Epo nor hepcidin was related to fetal growth parameters. Similar to adults, Epo was higher in the fetuses with the lowest iron stores (t-test), and hepcidin was directly related by regression analyses to fetal iron indices. Using fetal CRP as an index of fetal inflammatory activity because maternal CRP does not cross the placenta, we found by regression, that Epo was directly related to CRP, whereas hepcidin was not. With multivariate logistic regression, Epo was associated by CRP and iron status, perhaps reflecting that the fetal liver produces both Epo and CRP, and also stores iron. In contrast to adults, fetal Epo was unrelated to fetal hepcidin. By stepwise regression, hepcidin was also more strongly predicted by fetal iron status than maternal adiposity.
Based on previous work in models of diabetic pregnancies,24,25 it is logical that fetal overgrowth driven by insulin would also raise fetal metabolic rate, oxygen consumption and fetal Epo. Previously, higher fetal Epo levels in insulin-treated diabetic pregnancies were directly related to fetal growth parameters,8,25 presumably due to fetal glucose and insulin, augmented fetal metabolic rates, and fetal tissue hypoxia.24,25 This relationship between fetal growth and Epo was not evident in our study, perhaps because our study included only a subset with diabetes. However, focusing just on maternal adiposity, there was a strong relationship between maternal BMI and fetal Epo. Fetal Epo would have been primarily derived from the liver until production transitions to the kidney, ending several months after birth.9 This hepatic-to-renal transition is still a gap in current knowledge regarding fetal Epo regulation. Fetal Epo production, as in the adult, stimulates erythropoiesis as a functional response to sustain tissue oxygenation.26 Although fetal Epo was previously found to be higher after labored vaginal deliveries,26 we did not observe this effect, likely due to our cohort being healthy and lacking any perinatal complications as part of the recruitment strategy.
Fetal Epo levels were previously reported to be higher after maternal anemia,27 another relationship not found in our cohort, possibly due to early receipt of prenatal care and iron supplementation. Obesity is known to promote sufficient inflammation to raise hepcidin, block ferroportin, and limit enteral iron absorption in non-pregnant states28 and thereby potentially, in pregnancy, limiting the required 4-to 6-fold rise in iron absorption, potentially causing ID late in pregnancy.5 Considering that placental and intestinal iron transporters are the same, obesity may also directly hinder placental iron transfer to the fetus,3 as previously reported in diabetes.29 Because the site of Epo originates from fetal liver, as opposed to adult kidney, we are the first to report that, similar to adults,11 fetal iron status was associated with fetal liver Epo production, although we cannot definitely know that low iron was a cause or an effect of Epo production.
We found that fetal Epo was directly related to fetally-produced CRP, reflecting a potential response to fetal inflammation. Previously, fetal CRP was reported to rise in response to maternal complications of obesity including hypoxic sleep apnea, diabetes, or placental insufficiency-induced growth restriction.30 The CRP levels in our study were relatively low compared to those found after perinatal infections, but fetal CRP was still related to fetal Epo. Obesity increases the risk for gestational diabetes,1 but even in the absence of diabetes, fetal insulin and insulin-like growth factors can upregulate Epo production through greater fetal tissue oxygen demand.31 In adults, Epo may upregulate resting energy expenditure and block lipidogenesis, counteracting obesity10 and inflammatory responses.32,33 These novel Epo-induced effects should be further studied in fetal animals or human newborns. It would be intriguing to find a novel role for endogenous fetal Epo in fetal inflammatory metabolic disturbances.
In the opposite direction of the relationship between maternal hepcidin and maternal BMI,17 fetal hepcidin was indirectly related to maternal BMI, but in stepwise analysis, only fetal iron status predicted fetal hepcidin. This relationship between iron and hepcidin also appeared to be independent of fetal inflammation, even though inflammation is known to upregulate hepcidin production in adults.28 It is important to note that an appropriate fetal response (i.e., lower hepcidin levels) was found. The relationship between iron and hepcidin was also independent of fetal Epo, despite evidence that Epo administration downregulated hepcidin production in adults.18 Previous population-based data show that iron therapy improves maternal and fetal iron status,34 but this has not been studied in obese pregnancies.
In conclusion, depleted fetal iron status and subclinical inflammation, as indexed by CRP, was associated with Epo levels in fetuses of obese pregnancies. This novel relationship between Epo and CRP may also reflect a non-erythropoietic response by fetal Epo, potentially to modulate fetal inflammatory activity similar to that described for adults.35 Like in adults, Epo was linked to iron, but unlike in adults, Epo was not related to fetal hepcidin. Nevertheless, fetal hepcidin fell in response to fetal iron depletion. This fall in fetal hepcidin is reassuring because it supports the theory that the fetus would be responsive to therapeutic strategies intended to improve iron accretion. As importantly, our data add to the current understanding of the negative ramifications of maternal adiposity on the newborn metabolic state.
ACKNOWLEDGEMENTS
This work was supported by Meriter Foundation, National Institutes of Health (NIH 1 ULRR026011 UW CTSA Program, and NIH T32 DK077586 Pediatric Endocrinology Scholar), UW Education & Research Committee-Wisconsin Partnership Program, and Thrasher Research Fund.
The authors would like to thank the participating families, Meriter Hospital Birthing Center Staff and Blood Bank, Sue Shafranski, RN, Deb Krumpos RN, Patricia Green-Sotos RN, Vidya Sridhar MBBS, Melinda Chen MD, Beth Fischer PhD, Gregory Mayer PhD, Jens Eichman PhD, Chong Zhang BS, Sheila Roy, MD, Steven Marmer MS, Sheena Hirschfield BS, Patrick Halbach MS, and Karen Flores BS.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- 1.Schmatz M, Madan J, Marino T, Davis J. Maternal obesity: the interplay between inflammation, mother and fetus. J Perinatol 2010;30:441–446 [DOI] [PubMed] [Google Scholar]
- 2.Dixit A, Girling JC. Obesity and pregnancy. J Obstet Gynaecol 2008;28:14–23 [DOI] [PubMed] [Google Scholar]
- 3.Phillips AK, Roy SC, Lundberg R, Guilbert TW, Auger AP, Blohowiak SE, et al. Neonatal iron status is impaired by maternal obesity and excessive weight gain during pregnancy. J Perinatol 2014;34:513–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones AD, Zhao G, Jiang YP, Zhou M, Xu G, Kaciroti N, et al. Maternal obesity during pregnancy is negatively associated with maternal and neonatal iron status. Eur J Clin Nutr 2016;70:918–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Brien KO, Zavaleta N, Abrams SA, Caulfield LE. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr 2003;77:924–930 [DOI] [PubMed] [Google Scholar]
- 6.Lozoff B, Georgieff MK. Iron deficiency and brain development. Sem Pediatr Neurol 2006;13:158–165 [DOI] [PubMed] [Google Scholar]
- 7.Lesser KB, Schoel SB, Kling PJ. Elevated zinc protoporphyrin/heme ratios in umbilical cord blood after diabetic pregnancy. J Perinatol 2006;26:671–676 [DOI] [PubMed] [Google Scholar]
- 8.Lott DG, Zimmerman MB, Labbe RF, Kling PJ, Widness JA. Erythrocyte zinc protoporphyrin is elevated with prematurity and fetal hypoxemia. Pediatrics 2005;116:414–422 [DOI] [PubMed] [Google Scholar]
- 9.Dame C1, Fahnenstich H, Freitag P, Hofmann D, Abdul-Nour T, Bartmann P, et al. Erythropoietin mRNA expression in human fetal and neonatal tissue. Blood 1998;92:3218–3225 [PubMed] [Google Scholar]
- 10.Zhang Y, Wang L, Dey S, Alnaeeli M, Suresh S, Rogers H, et al. Erythropoietin action in stress response, tissue maintenance and metabolism. Int J Mol Sci 2014;15:10296–10333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kling PJ, Dragsten PR, Roberts A, Dos Santos B, Brooks DJ, Hedlund BE, et al. Iron deprivation increases erythropoietin production in vitro, in normal subjects and patients with malignancy. Brit J Haematol 1996;95:241–248 [DOI] [PubMed] [Google Scholar]
- 12.Sheffer-Mimouni G, Mimouni FB, Dollberg S, Mandel D, Deutsch V, Littner Y. Neonatal nucleated red blood cells in infants of overweight and obese mothers. J Am Coll Nutr 2007;26:259–263 [DOI] [PubMed] [Google Scholar]
- 13.Milman N, Agger AO, Nielsen AJ. Iron status markers and serum erythropoietin in 120 mothers and newborn infants: Effect of iron supplementation in normal pregnancy. Acta Obstet Gynecol Scand 1994;73:200–204 [DOI] [PubMed] [Google Scholar]
- 14.Ward DM, Kaplan J. Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta 2012;1823:1426–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rehu M, Punnonen K, Ostland V, Heinonen S, Westerman M, Pulkki K, et al. Maternal serum hepcidin is low at term and independent of cord blood iron status. Eur J Haematol 2010;85:345–352 [DOI] [PubMed] [Google Scholar]
- 16.Koenig MD, Tussing-Humphreys L, Day J, Cadwell B, Nemeth E. Hepcidin and iron homeostasis during pregnancy. Nutrients 2014;6:3062–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dao MC, Sen S, Iyer C, Klebenov D, Meydani SN. Obesity during pregnancy and fetal iron status: is Hepcidin the link? J Perinatol 2013;33:177–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, Cairns TD, et al. Erythropoietin administration in humans causes a marked and prolonged reduction in circulating hepcidin. Haematologica 2010;95:505–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo S, Frazer DM, Anderson GJ. Iron homeostasis: transport, metabolism, and regulation. Curr Opin Clin Nutr Metab Care 2016;19:276–281 [DOI] [PubMed] [Google Scholar]
- 20.McLimore HM, Phillips AK, Blohowiak SE, Pham DQ, Coe CL, Fischer BA, et al. Impact of multiple prenatal risk factors on newborn iron status at delivery. J Pediatr Hematol Oncol 2013;35:473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blohowiak SE, Chen ME, Repyak KS, Baumann-Blackmore NL, Carlton DP, Georgieff MK, et al. Reticulocyte enrichment of zinc protoporphyrin/heme discriminates impaired iron supply during early development. Pediatr Res 2008;64:63–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Committee To Reexamine IOM Pregnancy Weight Guidelines. Weight Gain during Pregnancy: Reexamining the Guidelines: Institute of Medicine; National Research Council; 2009 [Google Scholar]
- 23.Teramo KA, Widness JA, Clemons GK, Voutilainen P, McKinlay S, Schwartz R. Amniotic fluid erythropoietin correlates with umbilical plasma erythropoietin in normal and abnormal pregnancy. Obstet Gynecol 1987;69:710–716 [PubMed] [Google Scholar]
- 24.Hay WW Jr, DiGiacomo JE, Meznarich HK, Hirst K, Zerbe G. Effects of glucose and insulin on fetal glucose oxidation and oxygen consumption. Am J Physiol 1989;256:E704–713 [DOI] [PubMed] [Google Scholar]
- 25.Widness JA, Susa JB, Garcia JF, Singer DB, Sehgal P, Oh W, et al. Increased erythropoiesis and elevated erythropoietin in infants born to diabetic mothers and in hyperinsulinemic rhesus fetuses. J Clin Invest 1981;67:637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Widness JA, Clemons GK, Garcia JF, Oh W, Schwartz R. Increased immunoreactive erythropoietin in cord serum after labor. Am J Obstet Gynecol 1984;148:194–196 [DOI] [PubMed] [Google Scholar]
- 27.Erdem A, Erdem M, Arslan M, Yazici G, Eskandari R, Himmetoglu O. The effect of maternal anemia and iron deficiency on fetal erythropoiesis: comparison between serum erythropoietin, hemoglobin and ferritin levels in mothers and newborns. J Matern Fet Neonat Med 2002;11:329–332 [DOI] [PubMed] [Google Scholar]
- 28.McClung JP, Karl JP. Iron deficiency and obesity: the contribution of inflammation and diminished iron absorption. Nutr Rev 2009;67:100–104 [DOI] [PubMed] [Google Scholar]
- 29.Petry CD, Wobken JD, McKay H, Eaton MA, Seybold VS, Johnson DE, et al. Placental transferrin receptor in diabetic pregnancies with increased fetal iron demand. Am J Physiol 1994;267:E507–E517 [DOI] [PubMed] [Google Scholar]
- 30.Trevisanuto D, Doglioni N, Altinier S, Zaninotto M, Plebani M, Zanardo V. High-sensitivity C-reactive protein in umbilical cord of small-for-gestational-age neonates. Neonatology 2007;91:186–189 [DOI] [PubMed] [Google Scholar]
- 31.Kling PJ, Taing KM, Dvorak B, Woodward SS, Philipps AF. Insulin-like growth factor-I stimulates erythropoiesis when administered enterally. Growth Factors 2006;24:218–223 [DOI] [PubMed] [Google Scholar]
- 32.Woo M, Hawkins M. Beyond erythropoiesis: emerging metabolic roles of erythropoietin. Diabetes 2014;63:2229–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Di L, Noguchi CT. Erythropoietin, a novel versatile player regulating energy metabolism beyond the erythroid system. Int J Biol Sci 2014;10:921–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mei Z, Cogswell ME, Parvanta I, Lynch S, Beard JL, Stoltzfus RJ, et al. Hemoglobin and ferritin are currently the most efficient indicators of population response to iron interventions: an analysis of nine randomized controlled trials. J Nutr 2005;135:1974–1980 [DOI] [PubMed] [Google Scholar]
- 35.Alnaeeli M, Raaka BM, Gavrilova O, Teng R, Chanturiya T, Noguchi CT. Erythropoietin signaling: a novel regulator of white adipose tissue inflammation during diet-induced obesity. Diabetes 2014;63:2415–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]


