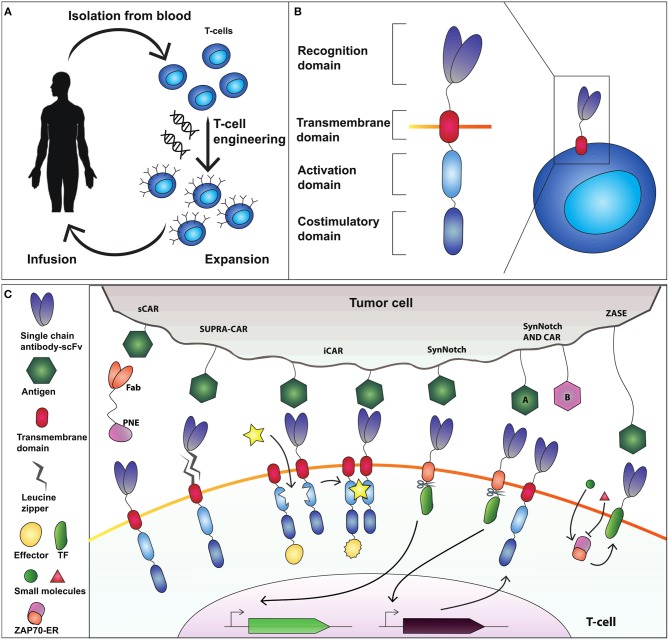
Figure 1.
Adoptive T-cell therapy and chimeric antigen receptor (CAR) design. (A) T-cells are isolated from the blood and genetically modified to express a cancer-targeting receptor. Engineered T cells are then expanded and transfused back into the patient. (B) The current CAR design comprises an extracellular recognition domain (a single chain fragment antibody-scFv for binding to the antigen) a transmembrane domain needed to anchor the receptor to the cell membrane, an activation domain (CD3ζ chain) and a costimulatory domain (commonly CD28 and 4-1BB). (C) Synthetic devices to improve specificity of CAR-T-cell activity. The sCAR system includes a switchable CAR (sCAR) and a Fab fragment fused to a yeast-derived peptide neo-epitope (PNE_tag). The Fab domain of the Fab-PNE module recognizes a tumor-associated antigen (TAA) whereas the sCAR specifically binds the PNE domain of the switch. This interaction activates sCAR-T cells against tumor cells. SUPRA-CAR is a two-component system consisting of a universal domain CARs expressing a leucin zipper extracellular domain (zipCAR) and a tumor-targeting scFv adapator (zipFv). The scFv of zipFv recognize the TAA while the zip binds the leucine zipper on the zipCAR acting as a switch. Also here it creates an immunological synapse that drives T-cell activation. iCAR consists of two incomplete CAR molecules that heterodimerize in presence of a small molecule which functions as a switch to activate T-cell response. SynNotch receptor comprises a scFv extracellular domain that recognizes the antigen, fused to a cleavable intracellular domain that retain a transcription factor (TF) to the membrane. The antigen recognition induces the cleavage of TF that translocate into the nucleus to activate output transcription. SynNotch/CAR AND-gate circuit to trigger activation when two distinct antigens are expressed on the target cell. SynNotch receptor activated by antigen “A” drives the expression of a CAR molecule which targets antigen “B.” Interaction with antigen B leads to T-cell activation. ZASE is a dual-gated ZAP70 protein switch, obtained by fusing Zap70 to the ligand-binding domain of the estrogen receptor ERt2. Two distinct small molecules, 4OHT (green circle) and 3-MB-PP1 (red triangle) turn, respectively ON and OFF protein activity.