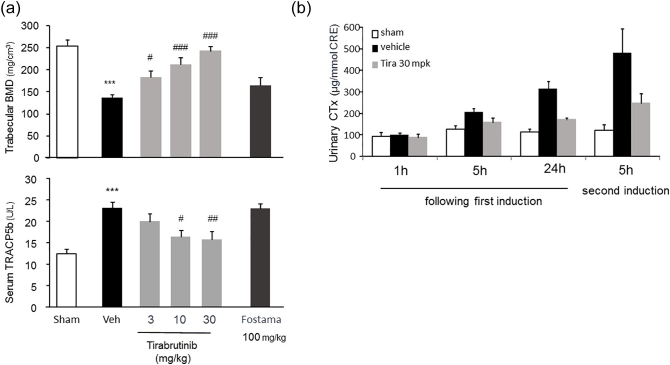
Fig. 4.
Effect of reproducibility of tirabrutinib and comparison with another BCR signaling modulator on RANKL-induced bone loss.
Tirabrutinib or fostamatinib at each dose was administered orally twice a day (5 times in total). At 1.5 h following the final induction, blood was collected and the femur was excised. (a) Bone mineral density of the distal end of the femur was measured by pQCT. Serum TRACP5b concentrations were measured by ELISA. Data are expressed as the mean ± standard error for 10 animals in each group. The t-test was used to compare the sham and vehicle group. Dunnett's test was used to compare the vehicle and tirabrutinib groups. ***: p < .001 vs Sham group. #, ##, ###: p < .05, p < .01, p < .001 vs group with vehicle. (b) Tirabrutinib at a dose of 30 mg/kg was administered orally twice daily (5 times in total). At 1, 5, and 24 h following the first induction and at 5 h following the second induction, urine was collected by compressing the lower abdomen of each immobilized mouse. Urinary CTx concentrations were measured by ELISA. Data are expressed as the mean ± standard error for 10 animals in each group.