Table 2.
Chemical modification of polyhydroxyalkanoate surface via different synthetic routes.
| Functional groups | Synthetic rout | Reaction condzition | Chemically modified PHA | Ref. |
|---|---|---|---|---|
| Mono-hydroxyl | Transesterification via acid catalyst | H2SO4, MeOH CH2Cl2, 100 °C | 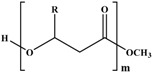 |
[66] |
| Di-hydroxyl | Transesterification via acid catalyst | 1,4-Butanediol APTS (para-toluene sulfonic acid monohydrate) CHCl3, 60 °C | 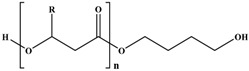 |
[67] |
| Transesterification via Dibutylene dilaurate catalyst | Ethylene glycol Diglym, 140 °C Dibutylene dilaurate | 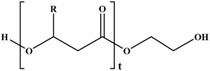 |
[68] | |
| Branched-poly(ethyleneimine) | Grafting via Michael addition | CHCl3 45–50 °C | 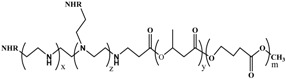 |
[69] |
| Carboxylic acid | Oxidation | Osmium tetroxide Oxone, BuOH, DMF 60 °C, 8 h | 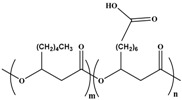 |
[70] |
| Epoxidation | Oxyrane addition | m-Chloroperbenzoic acid, CHCl3 20 °C, 12 h | 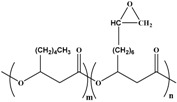 |
[71] |
