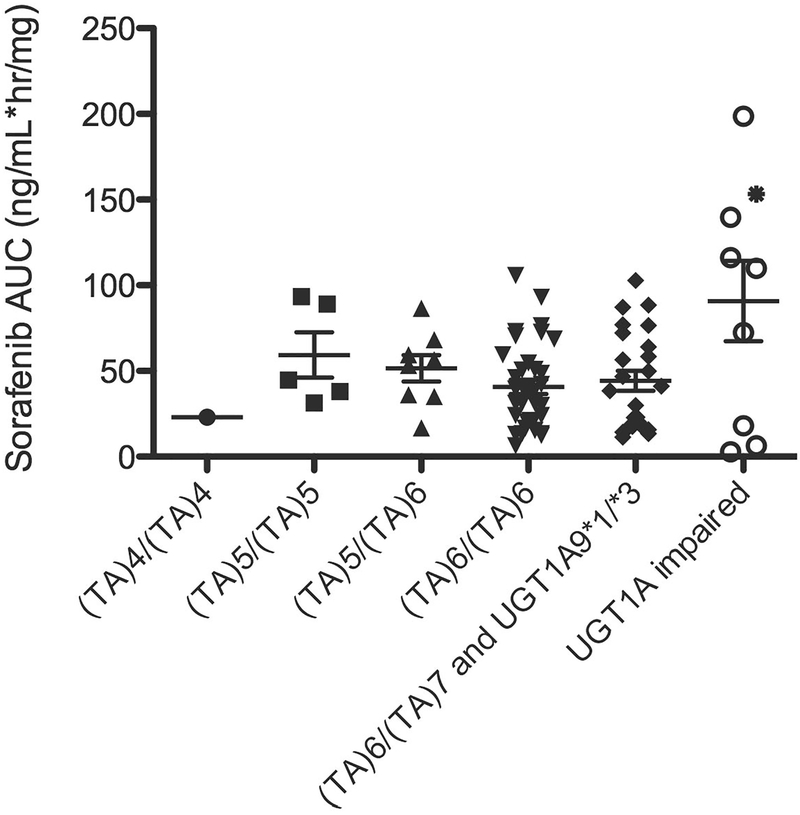
Figure 1.
Dose-normalized sorafenib AUC versus UGT1A genotype. UGT1A9*1/*3 carriers each also carried UGT1A1 (TA)6/(TA)7 (AUC = 17.3 and 20.2 ng/mL*hr/mg) while the single UGT1A9*3/*3 carrier also carried UGT1A1 (TA)6/(TA)6 (AUC = 153.3 ng/mL*hr/mg, as indicated by *). UGT1A-impaired implies patients with deficient UGT1A1 or UGT1A9 metabolism based on genetics. Excluded patients (n=38) are described in the supplementary results section and n=82 individuals were included in the present analysis. Of these, AUC data for n=8 patients participating on BAY-KS were not available; thus potential drug-drug interactions between ritonavir and sorafenib were not accounted for. There was no association between UGT1A1 and UGT1A9 genotype status when compared to sorafenib AUC (P = 0.20; Kruskal Wallis ANOVA).