Figure 8.
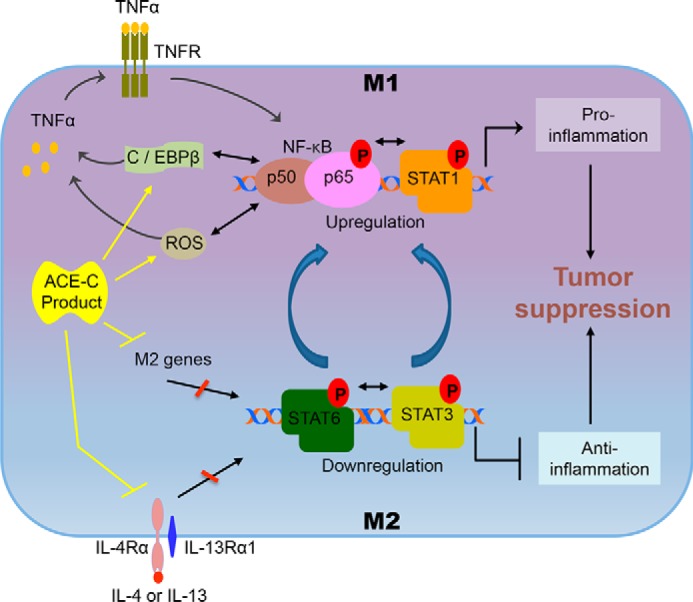
Effect of ACE C-domain up-regulation on macrophage polarization. The interaction of pathways for M1 and M2 macrophage polarization is outlined. By inducing anti-inflammatory signals, the M2 pathway is associated with tumor progression. This pathway is regulated by the activation of STAT3 and STAT6. In contrast, the M1 macrophage pathway suppresses tumor growth by inducing pro-inflammatory signals. The M1 pathway is mainly controlled by the activation of TNFα, NF-κB, and STAT1. The M1 and M2 pathways cross-talk with each other, which results in reciprocal modulation. NF-κB and STAT1 activation reduces STAT3 and STAT6 activation, or vice versa. As discussed in the text, TNFα plays a critical role in ACE C-domain–mediated M1 activation of macrophages and tumor resistance. Mechanistically, we found up-regulation of ROS and C/EBPβ in ACE C-domain overexpressing macrophages, which is known to enhance TNFα production. The enhanced level of TNFα can induce activation of NF-κB in a positive feedback loop leading to increased M1 signals and reduced M2 signals. ACE C-domain up-regulation also reduced expression of several key M2 markers including IL-4Rα, IL-4, IL-13, CCL17, CCL20, CCL24, and Retnla, which typically induce M2 macrophage signals and inhibit M1 macrophage activation, including up-regulation of TNFα expression. These data suggest that increased activation of TNFα, NF-κB, and STAT1, as well as decreased activation of STAT3 and STAT6, underpin the increased inflammatory phenotype induced by ACE C-domain up-regulation.
