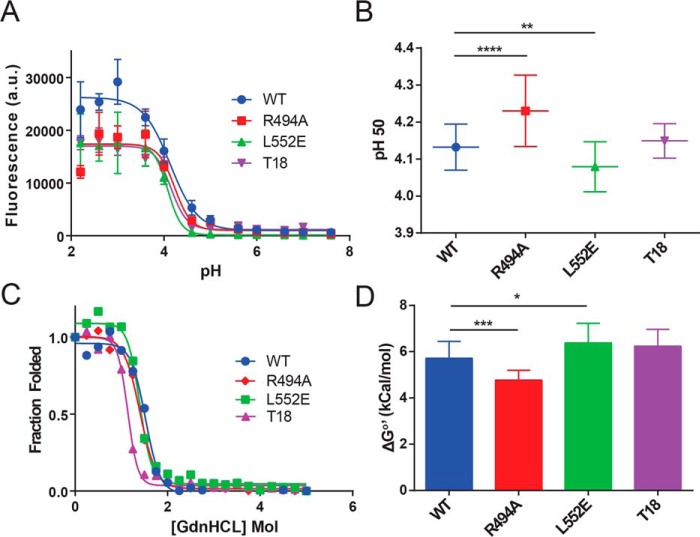
Figure 1.
Deimmunizing mutations alter the folding stability of PE-III. A, acid-induced denaturation of PE-III reported by Bis-ANS fluorescence (n = 3). B, analysis of best-fit pH 50 values; error bars indicate standard error; asterisks indicate significance by one-way ANOVA (*, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001). C, chemical denaturation of PE-III using GdnHCl and reported by intrinsic tryptophan fluorescence (representative of n = 3). The ratio of the intensity at a given [GdnHCl] to that at zero [GdnHCl] was plotted for each concentration, and the data were fitted to equations that solve for the free energy of unfolding at zero [GdnHCl] (ΔG0′). D, analysis of ΔG0′ values determined in C. Error bars indicate standard error, and asterisks indicate significance by one-way ANOVA.