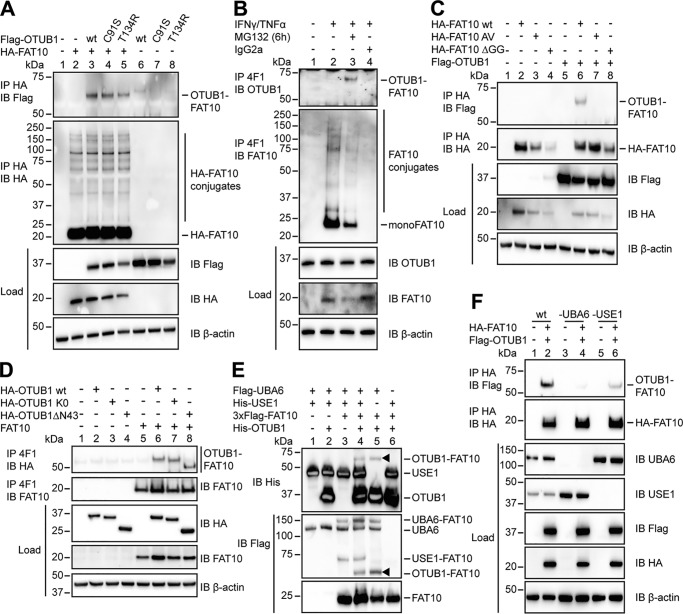
Figure 2.
OTUB1 becomes FAT10ylated in cellulo and in vitro. A, 24 h after transient transfection of HEK293 cells with the indicated expression plasmids, lysis in 1% NP-40 lysis buffer was performed. Cleared lysates were subjected to anti-HA immunoprecipitation (IP), and proteins were separated on 4–12% NuPAGE bis-Tris gels with subsequent Western blot (IB) analysis using anti-Flag M2 or anti-HA directly labeled antibodies. B, endogenous expression of FAT10 was induced by treating HEK293 cells with IFNγ and TNFα for 24 h. Where indicated, cells were treated additionally with proteasome inhibitor MG132 (10 μm) for 6 h. After lysis in 1% NP-40 lysis buffer, the cleared lysates were incubated with protein A–Sepharose and FAT10 mAb 4F1 or an unspecific IgG2a isotype control antibody. Western blot analysis was performed using a rabbit mAb against endogenous OTUB1 or a polyclonal rabbit antibody against endogenous FAT10. C and D, whole cell extracts from HEK293 cells transiently transfected with the indicated expression plasmids were treated and subsequently used in Western blot analysis as described in A and B. E, Western blot analysis of in vitro conjugation experiments where recombinant proteins (Flag-UBA6, 1 μg (0.1 mg/ml); 6His-USE1, 6 μg (0.6 mg/ml); 3xFlag-FAT10, 4 μg (0.4 mg/ml); and His-OTUB1 2.5 μg (1.25 mg/ml)) were incubated at 30 °C for 60 min, and the reaction was stopped by the addition of 5× gel sample buffer containing 4% 2-mercaptoethanol. Arrowheads indicate the OTUB1–FAT10 conjugate. F, HEK293 wt, HEK293 UBA6 KO, and HEK293 USE1 KO cells were transiently transfected with expression plasmids for HA-FAT10 and Flag-OTUB1. Subsequent lysis, immunoprecipitation, and Western blot analysis were performed as described in A. In all cellular experiments detection of endogenous β-actin was used as a loading control. One representative experiment of three experiments with similar outcomes is shown.