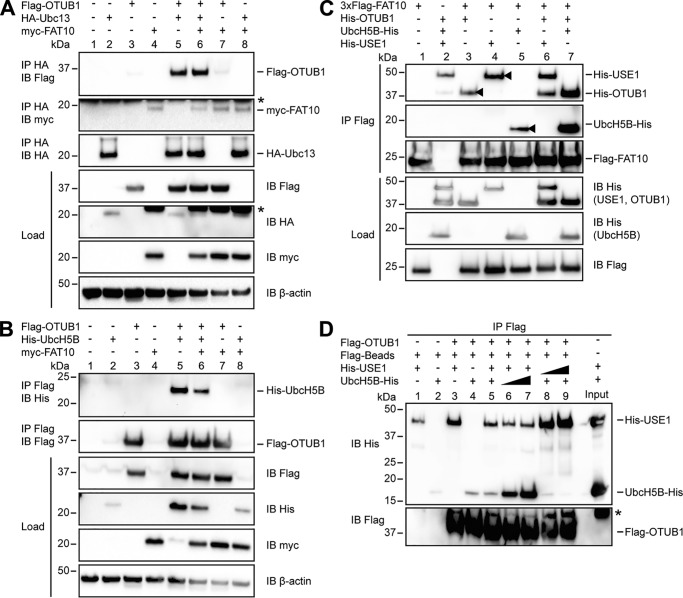
Figure 4.
FAT10 interacts with USE1, UbcH5B and OTUB1 in a direct, noncovalent manner. A, HEK293 cells were transiently transfected with Flag-OTUB1, HA-Ubc13, and myc-FAT10. After lysis in buffer containing 1% NP-40, the cleared lysates were subjected to immunoprecipitation (IP) using HA-coupled agarose. Western blot (IB) analysis was performed by using directly labeled antibodies reactive against HA or Flag or a mouse primary antibody against myc (clone 9E10). Endogenous β-actin was used as a loading control. B, extracts from HEK293 cells transiently transfected with the indicated expression plasmids were subjected to immunoprecipitation using Flag M2–coupled agarose. Western blot analysis was performed as described in A. Asterisks in A and B mark the remaining ECL signals. C, Western blot analysis of in vitro interaction experiments where equal molar ratios (8 μg) of recombinant proteins (3xFlag-FAT10 (0.4 mg/ml), His-OTUB1 (1.25 mg/ml), His-USE1 (0.6 mg/ml), and UbcH5B-His (0.6 mg/ml)) were mixed with Flag M2–coupled agarose and incubated for 60 min at 8 °C. Western blot analysis was performed by using directly labeled antibodies detecting His or Flag. The arrowheads indicate the direct interaction of FAT10 with OTUB1, USE1, or UbcH5B. D, transiently overexpressed Flag-OTUB1 was purified from HEK293 cells by immunoprecipitation using Flag M2–coupled agarose. For in vitro competition experiments increasing amounts of UbcH5B-His (8, 42, and 84 μg; 0.6 mg/ml) and His-USE1 (4, 22, and 44 μg; 0.6 mg/ml) were added to agarose-bound Flag-OTUB1 for 60 min at 37 °C. Western blot analysis was performed as described in C. One representative experiment of three experiments with similar outcomes is shown.