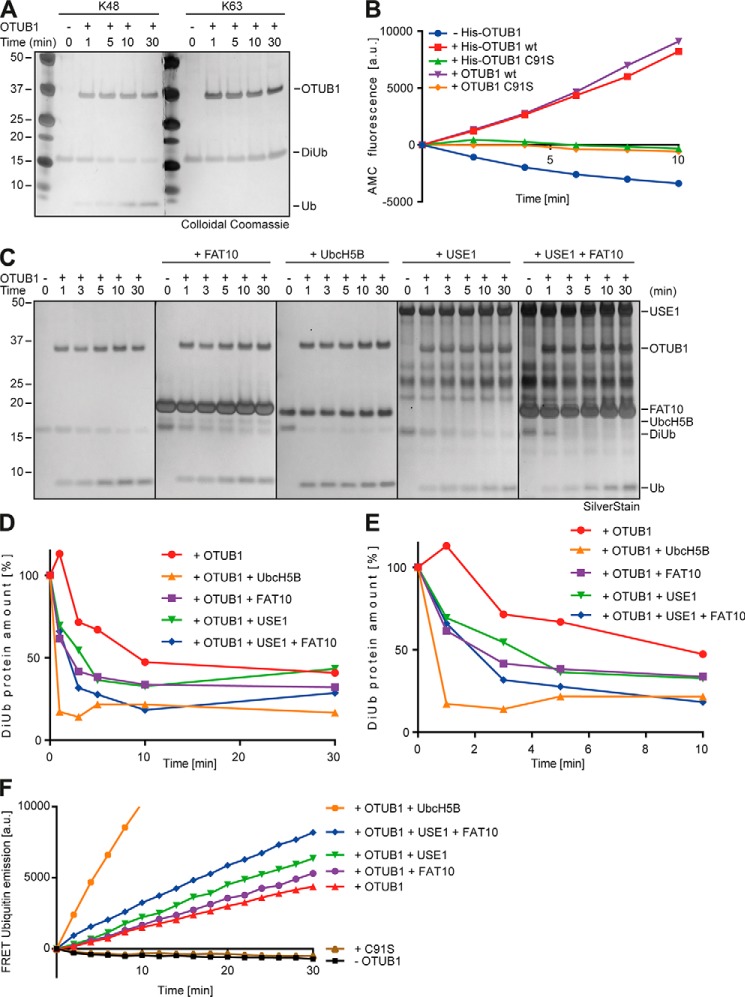
Figure 6.
FAT10 and USE1 enhance the cleavage activity of OTUB1 in vitro. A, recombinant Lys-48 or Lys-63 DiUb (5 μm) was incubated with tagless OTUB1 (5 μm) for the indicated time periods at 37 °C, and reactions were stopped by the addition of gel sample buffer supplemented with 4% 2-mercaptoethanol. Proteins were separated on 4–12% NuPAGE bis-Tris gels with subsequent colloidal Coomassie staining. B, quantitative analysis of AMC fluorescence (ex. 360 nm, em. 465 nm) of ubiquitin-AMC (5 μm) incubated with His-tagged or tagless OTUB1 WT or C91S (all 10 μm) for the indicated time periods at 30 °C. C, silver-stained gel of recombinant Lys-48–linked DiUb (5 μm) incubated for the indicated time periods with tagless OTUB1 (5 μm) and tagless FAT10, UbcH5B-His, and His-USE1 (all 10 μm) at 37 °C. The reactions were stopped by the addition of gel sample buffer supplemented with 4% 2-mercaptoethanol. D, densitometric analysis of cleaved Lys-48–linked DiUb by OTUB1 in the presence of FAT10, USE1, and UbcH5B. The DiUb protein amount at time point 0 was set to 100%. E, enlarged focus on the first 10 min of the densitometric analysis in D. F, FRET-based analysis for the cleavage of internally quenched Lys-48 DiUb (400 nm) by OTUB1 WT or C91S (both 30 nm) in the absence or presence of FAT10, USE1, and UbcH5B (all 5 μm). One representative experiment of three experiments with similar outcomes is shown.