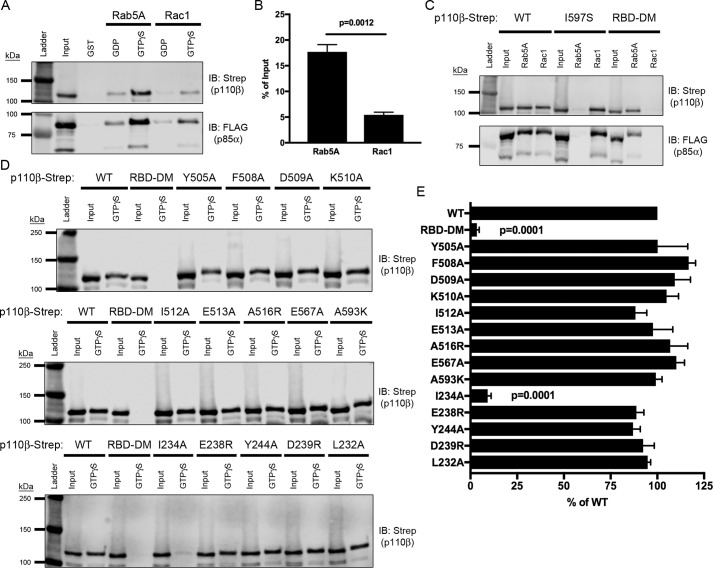
Figure 4.
Mutation of the Rab5-binding interface does not disrupt binding to Rac1. A, representative immunoblot (IB) of GST-Rab5A and GST-Rac1 pulldown assay. Lysates expressing WT p110β-Strep/p85α-FLAG were incubated with GST-Rab5A and GST-Rac1 beads loaded with nucleotide and assessed for binding via SDS-PAGE and immunoblotting for Strep and FLAG. B, quantification of PI3Kβ binding to GTPγS–Rac1 or Rab5A, expressed as a percentage of the input. Data represent the mean ± S.E. from three independent experiments. Statistical analysis was performed using an unpaired Student's t test. C, representative immunoblot of GTPγS-loaded GST-Rab5A and GST-Rac1 pulldowns incubated with lysates expressing p85α-FLAG and WT, I597S, or Rac1-uncoupled mutant (RBD-DM) p110β-Strep. D, GST-Rac1 binding assay with lysates expressing Rab5-uncoupled helical domain and RBD p110β mutants analyzed by SDS-PAGE and immunoblotted with Strep. E, quantification of the percent binding as compared with WT p110β. Data represent the mean ± S.E. from three independent experiments. Error bars represent S.E. in all panels. Statistical analyses were performed using one-way ANOVA. No statistically significant difference was observed, unless indicated.