Figure 1. MEK1 asymmetrically stimulates BRAF-KSR1 dimerization by interacting with KSR1.
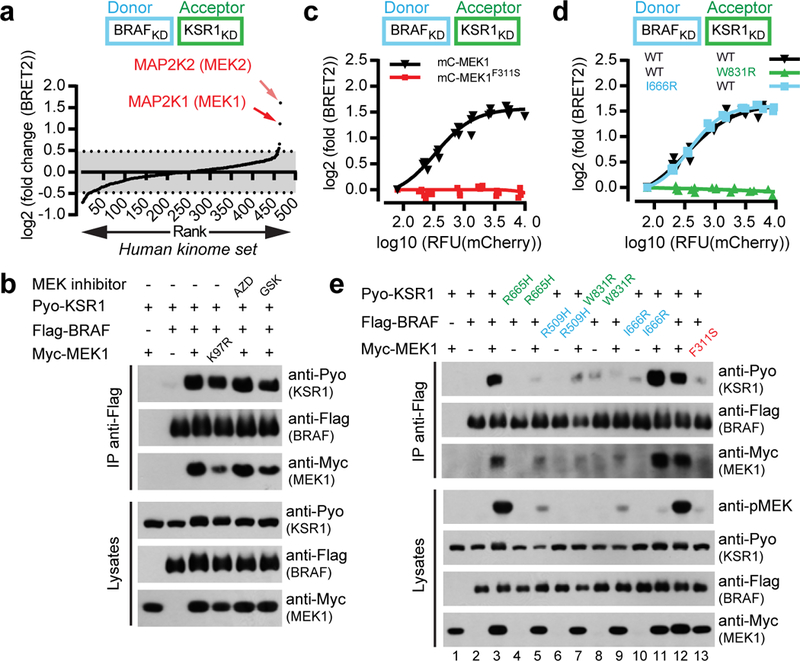
a, Whole-kinome screen identifies MEK1/2 as specific inducers of BRAF-KSR1 dimerization. BRET biosensors comprising RlucII-BRAFKD-CAAX and GFP10-KSR1KD-CAAX6 were used to screen 558 human kinase-related ORFs expressed from lentiviral vectors. b, Kinase-dead MEK1K97R or MEK inhibitors (AZD8330 and GSK1120212) (10 μM) did not alter MEK1-induced BRAF-KSR1 dimerization in co-IPs. c, mCherry-tagged MEK1F311S did not promote BRAF-KSR1 dimerization by BRET. d, Heterodimerization of BRAFI666R but not KSR1W831R was responsive to MEK1 expression by BRET. e, MEK1 promotes BRAF-KSR1 dimerization and BRAF transactivation by binding KSR1 in co-IP. Experiments in b-e were repeated three times. For gel source data, see Supplementary Fig. 1.
