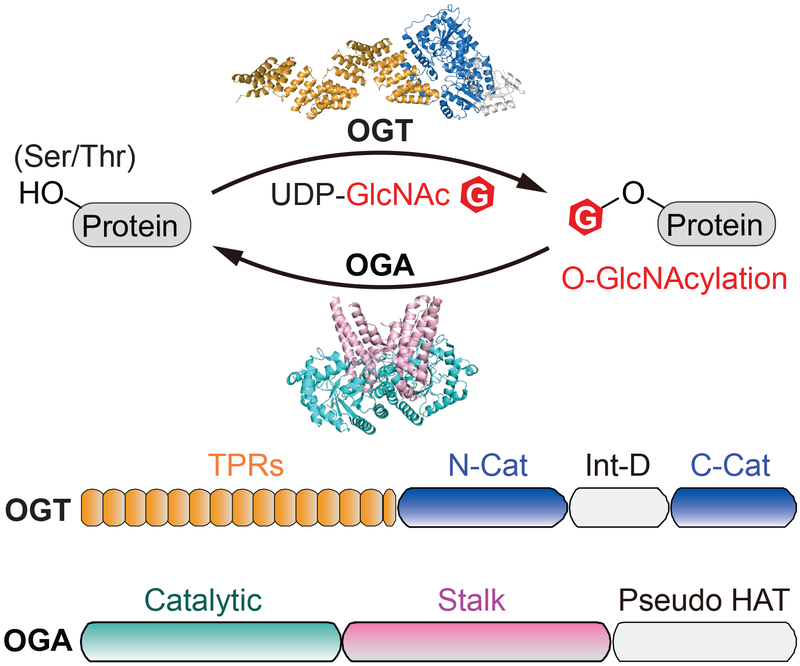
Figure 1.
Overview of O-GlcNAcylation and its cycling enzymes. Top panel: schematic of reversible O-GlcNAcylation. O-GlcNAc cycling enzymes, O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA), are represented by the full-length ncOGT model (generated from PDB 3PE4 and 1W3B) and a truncated human OGA structure (OGAcryst from PDB: 5TKE). Bottom panel: the schematic of domain architecture of ncOGT and OGA. OGT is comprised of a tetratricopeptide repeat domain (TPR, orange), catalytic domain (N-Cat and C-Cat, blue), and intervening domain (Int-D, light gray). OGA is comprised of a catalytic domain (Catalytic, cyan), stalk domain (Stalk, pink), and pseudo-histone acetyltransferase domain (Pseudo HAT, light gray).