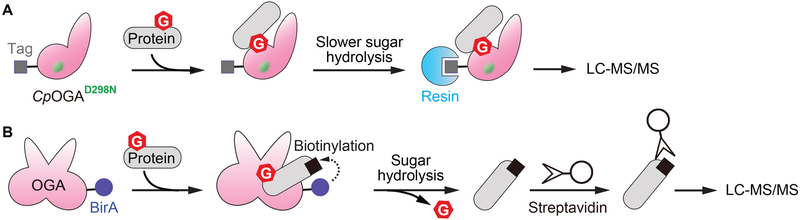
Figure 4.
Engineered OGA to enrich substrates and binding partners. A) Catalytically impaired bacterial OGA (CpOGAD298N) binds to substrate without quickly hydrolyzing the sugar. The interacting substrates can then be enriched by affinity purification of CpOGAD298N and identified by LC-MS/MS. B) The expression of an OGA-BirA fusion protein in cells results in proximity-dependent biotinylation of OGA-bound proteins, including OGA substrates. The enriched biotinylated proteins can then be detected by LC-MS/MS.