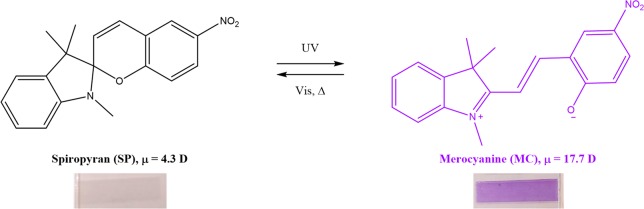
Figure 1.
Photochromism of 1,3-dihydro-1,3,3-trimethyl-6-nitrospiro[2H-1-benzopyran-2,2-(2H)-indole] (SP). Upon exposure to UV, the purple merocyanine (MC) is formed, while upon exposure to visible light and/or heat, the SP spiropyran is obtained again. Top: isomerization of SP and MC molecules.19 Bottom: photographs of drawn UHMW PE tapes containing SP before and after UV light irradiation.