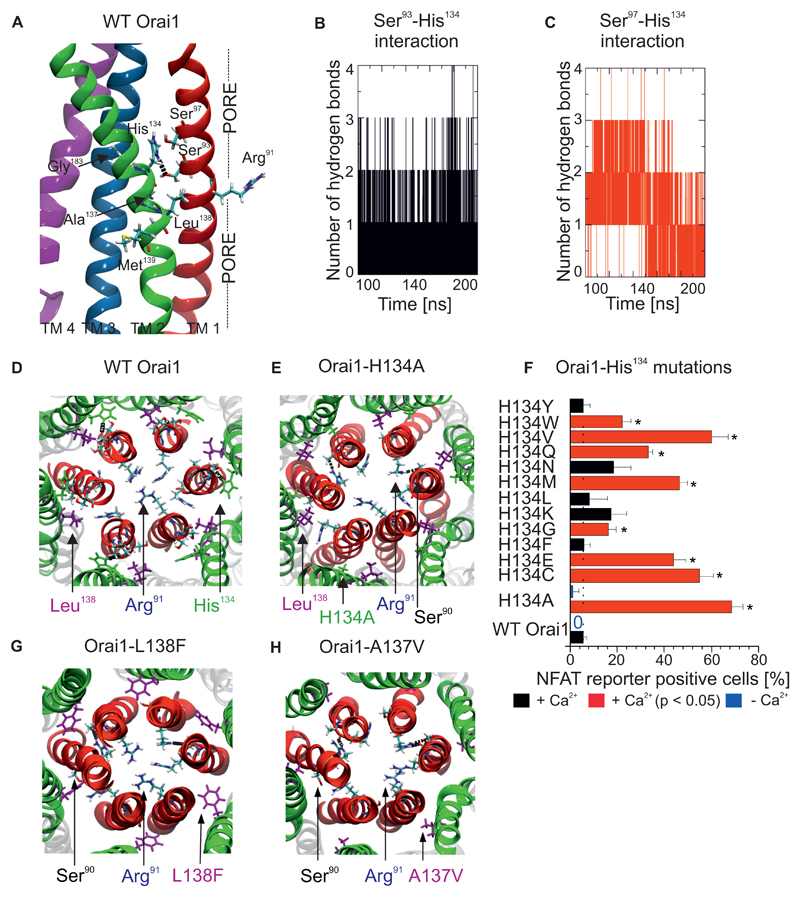
Figure 2. Interaction between a central TM2 segment and the pore helix controls Orai1 channel gating.
(A) Details of the central TM2 segment in Orai1 mutants, including pathophysiological and cancer-associated mutations (A137V, L138F, M139V), near TM1 and TM3 (Gly183). A hydrogen bond is observed between His134 and Ser93. (B,C) The number of hydrogen bonds between (B) Ser93 and His134 and (C) Ser97 and His134 in wild-type Orai1 as determined by molecular dynamics simulations. (D) Top view of a representative image of molecular dynamics simulations of the wild-type Orai1 pore, with the side-chains of Arg91, His134 and Leu138 highlighted. (E, G, H) Similar snapshots are shown of molecular dynamics simulations of Orai1-H134A (E), Orai1-L138F (G) and Orai1-A137V (H). (F) Percentage of RBL cells that express NFAT-driven RFP (average + SEM), measured 24h after co-transfection of the NFAT reporter and various Orai1 His134 mutants or wild-type Orai1. Cells were in a bath solution containing 2 mM Ca2+. Significantly increased number of cells (t-test, p < 0.05) positive for NFAT-driven RFP are indicated by a red bar and a star (*). Analogous experiments were performed in a nominally Ca2+-free bath solution to detect the co-expression of the NFAT reporter and wild-type Orai1 or Orai1-H134A, indicated by blue bars (n = 3 – 5 cell images, each from a separate transfection).