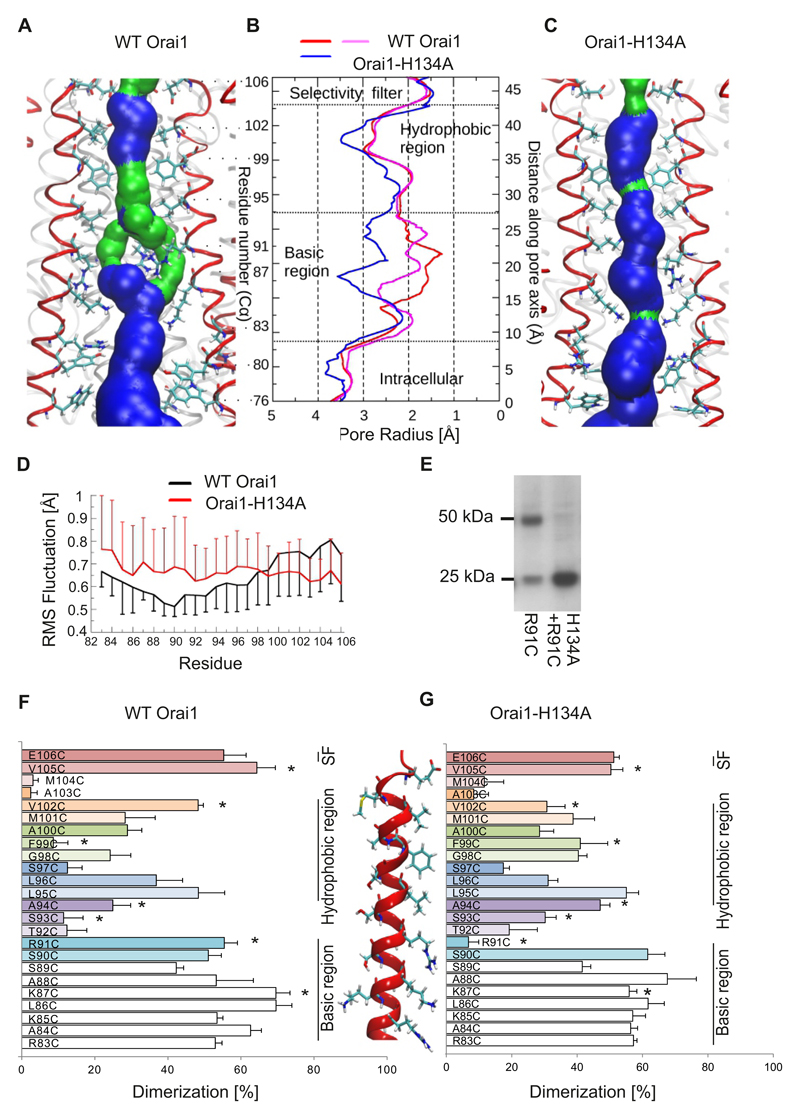
Figure 5. Increased pore size in the open conformation of Orai1-H134A channels.
(A,C) Representative snap shot (time point at 192 ns) of the equilibrated part of 200 ns long molecular dynamics simulations for wild-type Orai1 (A) or Orai1-H134A (C) showing the pore forming TM1 helices (4 out of 6 TM1 helices shown in red) and pore lining residues from Glu106 to Trp76. The pore surface is shown in blue (radius > 1.15Å) and in green (radius is between 0.6 to 1.15Å). (B) Average pore radius of wild-type Orai1 (red and magenta) and Orai1-H134A (blue) for a 2 ns bin corresponding to the time points shown in A and C. (D) Fluctuations of individual TM1 residues of (black) wild-type Orai1 and (red) Orai1-H134A are measured as the root mean square (RMS) deviation of Cα atom for the last 50 ns of the 200-ns-long molecular dynamics simulations. (E) Representative western blot of a crosslinking experiment overexpressing Orai1-R91C and Orai1-H134A-R91C showing monomer and dimer formation. (F, G) Dimerization efficiency in (%) of cysteine crosslinking for engineered cysteines (R83C to E106C) in the (F) wild-type Orai1 or (G) Orai1-H134A TM1 pore segment. A cysteine-free Orai1 background was used. For each cysteine position, parallel experiments (n = 5 - 8 individual transfection for each cysteine position) with wild-type Orai1 and Orai1-H134A background were performed on the same day, and significant differences (t-test, p < 0.05) are indicated by a star (*).