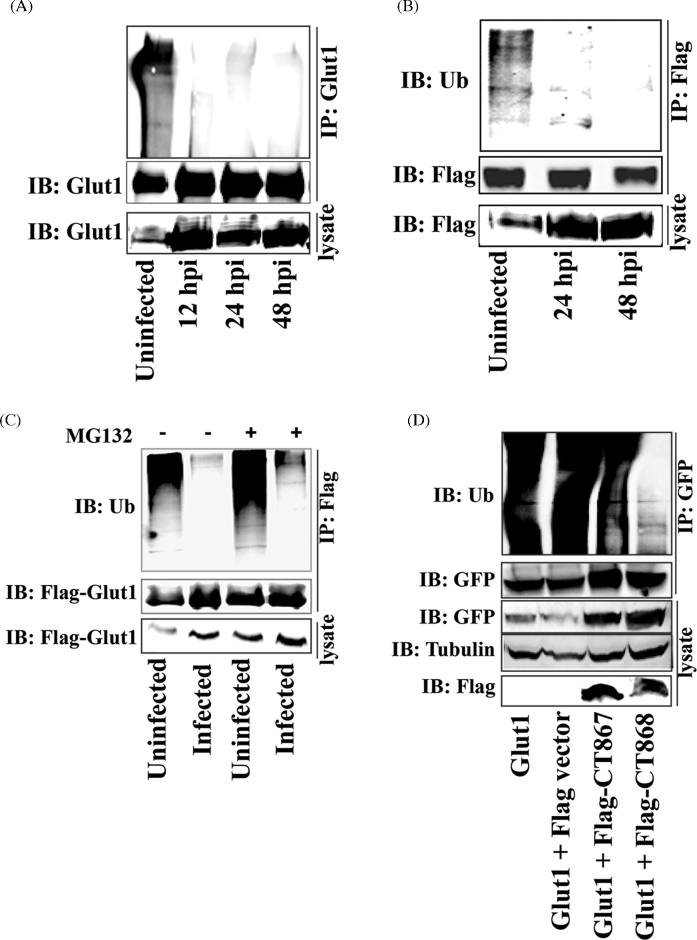
Figure 6.
Stabilization of GLUT1 by CT868-mediated deubiquitination during the chlamydial infection. (A) Hela cell monolayers were infected with C. trachomatis for 12, 24 or 48 h. Endogenous GLUT1 from infected cell lysates was immunoprecipitated using rabbit anti-GLUT1 antibody and protein A/G Magnetic beads, and then immunoblotted and probed with rabbit anti-ubiquitin and rabbit anti-GLUT1 antibodies. (B) Hela cells overexpressing Flag-GLUT1 were infected with C. trachomatis for 24 or 48 h. Flag-GLUT1 from cell lysates was immunoprecipitated using mouse anti-Flag M2 antibody-conjugated beads, and then immunoblotted and detected with anti-ubiquitin and anti-Flag antibodies. The expression of Flag-GLUT1 from cell lysates was immnoblotted and detected by anti-Flag antibody. (C) Hela cell monolayers expressing Flag-GLUT1 were infected with C. trachomatis for 20 h, and then treated in the presence or absence of MG132 (10 μM) for 4 h. GLUT1 from cell lysates was immunoprecipitated using mouse anti-Flag M2 antibody-conjugated beads, and then immunoblotted and stained using anti-ubiquitin and anti-Flag antibodies. (D) Hela cell monolayers stably expressing GFP-GLUT1 were transfected with p3xFlag-CMV vector, Flag-CT867 or Flag-CT868 expressing constructs. At 30 h post-transfection, GLUT1 from cell lysates was immunoprecipitated using anti-GFP antibody and then immunoblotted and stained with anti-ubiquitin and anti-GFP antibodies. The expression of CT867 and CT868 from cell lysates was detected by immunoblotting using anti-Flag antibody. All of the experiments were repeated at three times and a representative data was shown in each experiment.