Figure 1.
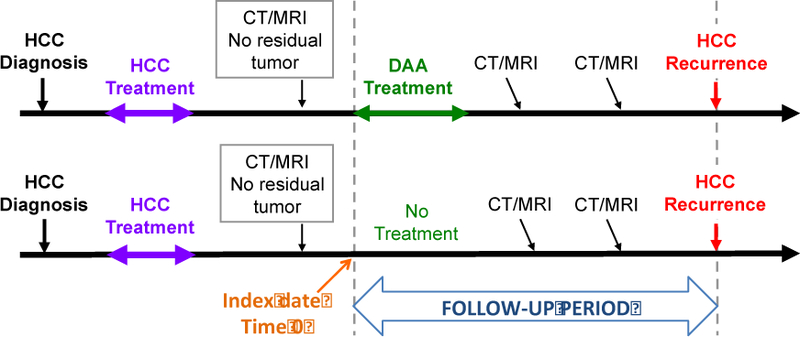
Proposed study design for evaluating the risk of recurrent HCC associated with DAA treatment. Follow-up time begins at the start of antiviral therapy for treated patients and at the “Index date” for untreated patients. The index date is chosen in untreated controls to match the date of DAA treatment initiation in their matched treated patients, i.e. to be an equal duration of time from the time of HCC treatment. Both treated and untreated patients need to have evidence of lack or residual tumor prior to the initiation of DAA treatment (in the treated patients) or the index date (in untreated patients). This accounts for immortal time bias, that is, the bias related to the fact that DAA-treated patients cannot possibly have had HCC recurrence prior to DAA treatment and that almost all patients with early recurrence will, necessarily, be in the untreated group unless a matching scheme such as the one shown is used. Furthermore, the comparison between treated and untreated patients has to be adjusted for baseline characteristics that are associated with tumor recurrence, especially tumor burden and type of tumor treatment. Finally, treated and untreated patients need to have similar and adequate methods of surveillance for HCC recurrence during the follow-up period.
